Research Article - (2021) Volume 0, Issue 0
A Formation of the alfalfa Entomofauna
A.D. Rixsikhodjaevna1*, R.A. Yusupovich2 and H.P. Bobakulovna1Abstract
The paper involves data on alfalfa entomofauna, which was composed of the composition of entomocomplex of alfalfa, including 207 species of phytophagous and 159 species of entomophagous. The formation of the entomofauna of alfalfa agrobiocenosis was detected in alfalfa of different years. The nutritional specialization and nutritional characteristics of the phytophagous and entomophagous were determined.
sushihouse kolstads americanpridefasteners trueforge hotelposeidon youngswoodfinishing arditomason thebestofreno eeclongisland doktervanhecke famsales adla bogatylaw prefplastics kirbycontractinginc unlsp boban dottoressaromolimonica thebestofsanfrancisco vernix digitaldocuments tristatepropertybrokers sotrafib cemcorpny agenziaimmobiliarebuti joesitalianfoodmarket corpoguardiedicitta icsatc antoniniassicurazioni besel strappedincarseatsafety drevobeton blessingconstructionny centre-endorphine qmbtunisia elearning 357 mpress rrappliance ciaautomazioni antikva stubbanvel tacticalpublicrelations mpulshnk paulyboybrand lordshoes ijd-procom thebestrestaurants atkpalvelut ceat leonfukspc pilotexamssa ashgrovecabins universalshielding thebestofcharlotte johnjmazurinc rollnroaster thecabinetwarehouse pocketchangeduo thebestofmilwaukee resan sooli vwe centralwindowcleaning reinforcedplasticslab bugbustersofli ciandrigiardini arfada centrumeigenwijs thebestofmemphis maisonpearly islipll dcgraphicsinc agetranquille relisandroth thebestofcharleston traiteur-wn safi-ingenierie justmyvoice harrishardware louisbarbatolandscaping brightstartoursworld rga-insurance calacatajumper ezantia mrcheapocds van4holiday cigap richtour fagyhatar sicc weshopmall ventovuori sobatrapcapbon unityrubberllc thebestofsaltlakecity ircinc sescoindustries connply storen-servicesenter psnry greatneckcollision ironfitendurance tuscanycountryhouse sansovinocalcio bbradydesign samsam vdtarification springersoil thebestofdayton milantechnology labradoodlesoflongisland daliamohamed theconsultantpowerhouse gunnbrush kruunuosk shulmanproduce balcosupply conso-med federalnetworks bellmoreglass alwaysaffordableconcrete jayteeinsurance carolina-cabins cmorfinance stretchritepackaging dishaairwaysenterprise biocontrol distribio peterivill lcc inspekta jvidesigns royalroseinc calabriapizza fadhila prato-pronto-effetto arbemachine olsonelectricnj tkbl tuttifotokft alpernmd ggsupplywholesale almaxcorporation sotuflex ecustomgutter studenipotok metal-lineconcept rettsodontologi nutecsystems aclotbeach gjonnes-bygg cavalierinternational mnemos southfloridashuttles ajchemicalsupply rachelsfireisland concepto nesponge ultimatestylesofamerica techniquesmadeeasydrivingschool stevesmeatsfreeport eastwest gritbrush plussplan biosens viltkam wikaya paintballconcept justmyvoice securecarkeysupply justmyvoice allcountylegal catt plugandcharge ttandlcontracting justmyvoice islandboatlettering gms-tunisie sportsiena bloeiop touchofclasscollision kotekservice ittoscana pallongislandlacrosse industrialfinishings painoutband rakvag-batforening konemies rrfamilychiropractic infienile shbcgroup footpharmacydirect scuolaguidaprato ans-nettoyage autoskola saafa autolaky1 fixcars longislandelitelandscaping rayscan palaconstruction studio44 crealhome viniferi gavinburke ciprianigiardini davidpokorny osteriailcapodaglio thebestoffairfax centroorafofaccioli ateliervb bbdps thebestoffresno prodigus stratpak umisushirestaurant sotim suldalrenovasjon amer-equip k-kleven jerryspridepotatoes neuroky psicologozampoli jjslandscaping thebestofoklahomacity responsivesales paratie-antiallagamento-shop hearproof cfat ruspinameubles planetbioplastics lynbrook-plumber fcdf-ye brechanparkett valley-stream-plumber diagnosismaker aquavaria jedit garageennour heimdalbygg thebestoflittlerock raybomarine bayshorepaper gmstowing potatura-abbattimento-piante thebestoflouisville michaelalbert thebestofannarbor unitypavers mtnfueloil durub-mudiya lesgensdere dormerking thusney italianvistatravel maiemad gtiuniformcleaning suddenimpactli levituuli sourisalavie mayoiltank mschwartzfeather rands arieslimousines orthoticworld longislandcocktailhours waterjet allislandpaving dukediagnostic klfgoteborg yanezviaggi apruk decogato springeroilltd semapsolar irisgioiellicomprooro msedpsoftware fourcmanagement holemans 7consulting medinet evertile robertwitcomblandscape ceramics adrobotengineering volt-energy huisjacobs chimneyserviceboston eastmainstdental gcbt rememberingbriank elligiardiniespurghi paulslandscaping luisrestorations mgstunisie fixcarsny crcdd hotelprincipessalucca turvahallinta ralphjr thales irrigazione-giardini spantecsystems biomedic fgt-trading sols-egypt spectrumlaboratoriesinc prosecurite spongewarehouse afsainc dellafrancadevelopmentgroup leragazzedifirenze ferrettiwatches dovreentreprenor digitalhvac mostlymica fleurs-velghe autoscuolalebadie interlockingrubbertiles meadowcreekhoa bcn corpjetsupport jrkitchensflooring arteletti
Keywords
Alfalfa, insects, phytophages, entomophages, parasites, nutritional specialization, polyphages, ligophages, monophages.
Introduction
Alfalfa is an extremely valuable legume and plays an important role in terms of nutrition, agrothecnic, melioration, and phytosanitary. However, because alfalfa is infested with many invertebrates at different stages of development, not only is its large yield lost but also nutrients are drastically reduced. Importantly, alfalfa agrobiocenosis is aimed at maintaining natural entomophagous populations, creating favorable conditions for their reproduction and attracting them to the protected field, reducing the number of pests of alfalfa and other agricultural crops, preventing their mass reproduction. Because alfalfa agrobiocenosis is the primary source of beneficial insects (entomophagous, plant pollinators), it is also important to enrich agrocenoses against natural pest populations of high-yielding entomophagous against pests of other agricultural crops (Ellington et al., 1997; Hoch et al., 2001). In order to study the process of formation of the alfalfa agrocenosis entomofauna in the country, we carried out calculations on one-year, two-year and three-year alfalfa fields.
According to the nutritional properties of common phytophagous in annual alfalfa, monophages involve 7.9%, including Sitona longulus is dominant species, oligophages are 15.7%, Sitona cylindricollis is dominant species, polyphages are about 77.4%, as the dominant species we can note Acyrthosiphon pisum Therioaphus trifolii, Lygus pratensis, and they were recorded (Khamraev et al., 2000; 2001).
Materials and Methods
To study the ways of the formation of alfalfa entomofauna, accounting work was carried out on alfalfa and seedbeds of different ages (first, second, third, and more). The density of dominant species in alfalfa and weeds was determined by K.K. Fasulati method. We determined the population density according to the following formula:
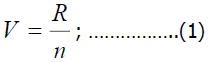
Here, V -the population density;
R-the total number of species in all samples;
N-the number of taken samples;
The occurrence of the species was studied according to the following formula.
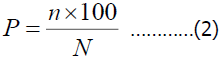
where n is the type of sample found;
N-the total number of samples taken;
P- meeting
Dominance (predominance), relative abundance was determined in relation to the number of breeds of common species;
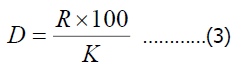
Here, D-dominance
K-the sum of species of species in all samples;
R-the sum of the species.
Calculations were also performed on plant residues using the soil sampling method (at least 32 samples measuring 25 × 25 cm in each variant).
By the method of F.M. Uspensky, the quantitative number of harmful species in foreign plants was calculated on average 1 m2 depending on the stages of development.
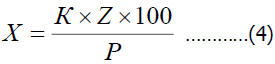
In this case X-the number of pests per 1 m2 area;
K-average number of weeds per 1 m2;
R-the total number of plants considered;
Z-the number of plants on which the pest is spread.
The number of wild arthropods in alien plants was determined according to the stage of development.
The species composition of weeds in irrigated agricultural areas was studied using a weed identifier (Khamraev, A.Sh., 1999).
Results and Discussion
Distribution of phytophagous plants by level of specialization in nutrition in one year (%)
According to nutritional specialty, 26.3% of the total phytophagous is fed on the roots of alfalfa, the dominant species are Agriotes meticulosus and Sitona cylindricollis. The productive organs of the plant are infested with 7.3% phytophages; the dominant species are Lygus pratensis, L.gemelatus, and Adelphocoris lineolatus.
According to the nutritional properties of phytophagous, it is composed of 13.6% monophages in two-year alfalfa, the dominant species are Sitona longilus, Phytonomus variabilis; oligophages are 20.7%, Sitona cylindricollis, Agromyza mint are dominant species; polyphages are 65.7%, Austroagallia laevis, Cicadella viridis, Empoasca meridiana, Aphis crass, Lygus pratensis, Adelphocoris lineolatus are the dominant species.
Nutritional distribution of phytophagous in 2-year-old hay alfalfa (%)
Depending on the specialty of nutrition, 60.23% of phytophages feed on alfalfa leaves, twigs and stems, so Phytonomus variabilis, Sitona cylindricollis, Aphrodes fergansis, Cicadella viridis, Empoasca meridiana, Acyrthosiphon pisum, Heliothis viriplaca and Heliothis viriplaca aredominant species, 9.3% of phytophages feed on the root system of alfalfa, Sitona cylindricollis, S.longulus, Agriotes meticulosus are the dominant species, 14.7% of phytophages are fed with alfalfa organs (combs, flowers, and legume), Lygys pratensis, L.gemellatus, Adelphocoris lineolatus are the dominant species and 16.77% of other phytophagous insects feed on the rests.
Nutritional distribution of phytophagous insects in 2-year-old hay alfalfa (%)
According to the nutritional characteristics of the three-year-old alfalfa, 10.7% of the phytophages are monophages, Phytonomus variabilis and Sitona costipennis are the dominant species, 12.11% are oligophagous, Sitona cylindricollis and Agromyza mint are the dominant species, 77.29% are polyphagous, Calliptamus italicus, italicus, Cicadella viridis, Empoasca meridiana, Aphis craciv pratensis, Thrips tabaci are the dominant species.
Distribution of phytophages according to nutritional properties in 3-year-old hay alfalfa (%)
Depending on nutritional characteristics, 60.86% of alfalfa stems, twigs, and leaves are infested by phytophagous, so Phytonomus variabilis, Sitona cylindricollis, Aphis craccivora are dominant species. 6.89% of the alfalfa roots are infested by phytophages; Gryllotalpa gryllotalpa and Agriotes meticulosus are dominant species. 16.1% of pests are fed by the productive organs of alfalfa, and here Lygus pratensis, L.gemellatus, and Adelphocoris lineolatus are the dominant species.
Distribution of phytophagous on nutritional characteristics in 3-year-old hay alfalfa (%)
According to the nutritional characteristics of biennial alfalfa, monophages-28.2%, Phytonomus variabilis, Bruchophagus roddi are dominant species, oligophages-31.1%, Therioaphis trifolii and Sitona cylindricollis are dominant species, polyphages-40.7%, Lygus pratensis, L.gemellatus, Adelphocoris lineolatus, Thrips tabaci are the dominant species.
Distribution of phytophagous on nutritional characteristics in 2-year-old seed alfalfa (%)
Depending on nutritional characteristics, 61.9% of phytophages feed on alfalfa stems, twigs and leaves, Aphis craccivora, Therioaphis trifolii, Phytonomus variabilis are dominant species, 15.5% of phytophages feed on the root system with Sitona cylindricallis and S. longulus are dominant species, 22.6% of phytophages feed on plant reproductive organs with Lygus pratensis, L.gemellatus, Adelphocoris lineolatus, Bruchophagus roddi are the dominant species.
Distribution of phytophages according to nutritional characteristics in 2-year-old alfalfa (%)
Omnivorous pests are dominated according to the nutritional nature in the annual alfalfa, we observed an increase in the number of oligophagous and monophagous in biennials. In seed alfalfa, although the number of oligophages and monophages is significantly increased, the number of polyphages predominates.
According to nutritional specialization, phytophagous is dominated in alfalfa of all years that feed on the stems, twigs, and leaves of the plant. We observed an increase in phytophages in annual alfalfa due to the large number of species of the family Cicadellidae. In both two- and three-year-old alfalfa, representatives of the same family made up the majority, and the growth of specialized species of families such as Curculionidae, Miridae was found.
The number of phytophages feeding on the roots of alfalfa is high in annual alfalfa (26.3%), a sharp decrease in phytophages was observed in alfalfa of two years (9.3%) and in alfalfa of three years (6.89%), which is directly related to the thinness of the roots.
In experiments, we studied the specialization of the food of species that live in wild plants as a source of pest formation. Among phytophages, we studied representatives of the Aphididae family to feed on a wide variety of plants, including alien plants belonging to 11 families (Gramineae, Compositae, Legiminosae, Chenopodiceae, Cruciferae, Solanaceae, Betulaceae). Tetranichus urticae feeds on representatives of five families (compositae, Cruciferae, Leguminosae, Solansea, Chenopodiaceae).
We determined the nutrition of phytophages in various plants. Representatives of Tenebrionidae, Acrididae, and Gryllidae feed on five family plants (Gramineae, Compositae, Leguminosae, Chenopodicea, and Malvaceae); Representatives of the families of Miridae, Pentatomidae, Thripidae, and Chrysomellidae feed on plant species belonging to three families of wild plants.
During our investigation, we identified 59 plant species belonging to 17 families of wild plants that grow near the edges of the field. In terms of species composition, the Gramineae family includes a more extensive species (17 plant species), 12 plant species belong to the Campositae family, and 5 plant species belong to the Chenopodiaceae family. Three plant species from both Amaranthacea and Cruciferae families of Amaranthacea, Cruciferae were defined, 1 plant species from each family was recorded, such as Papavereceae, Ceratophyllaceae, and Plantaginaceae families.
Representatives of the families Cicadellidae, Acrididae, Buprestidae, and Aphididae dominated in terms of the number of species in the diet. As phytophages emerge, entomophagous also begin to migrate to alfalfa. Representatives of the families Coccinellidae, Chrysopidae, and Syrphidae are common in alfalfa, which is used for different purposes (hay, seeds) in different years.
The formation of harmful entomofauna in annual alfalfa is directly related to the amount of nutrients. Due to the instability of the plant phytomass, all-consuming pests predominate. These species feed on weeds that grow in the field and in the alfalfa. Later, due to the formation of plant cover and the interaction between insects and the plant, oligophages begin to displace all species.
As the ecological environment stabilizes in the crop, monophages begin to displace oligophages. This is because old alfalfa is the source of monophagous, but some monophages that later migrate to alfalfa than oligophagous and polyphagous will later become the dominant species. Because alfalfa is their favorite plant, it leads to rapid multiplication and development of monophages due to favorable environmental conditions in the crop.
11 genera, 5 subgenera, 43 families, 158 seeds, and 207 species of phytophagous were recorded in the areas studied. The identified species were classified according to their nutritional characteristics and nutritional specialization (Table 1).
| Name | Occurrence | Nutrition | ||
|---|---|---|---|---|
| In alfalfa | In wild plants | Feature | Specialty | |
| 1 | 2 | 3 | 4 | 5 |
| Type Mollusca | ||||
| Class Gastropoda | ||||
| Genera Stylommatophora | ||||
| Siccinea putrus L. | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Vallonia pulchella Mull. | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Type Arthropoda | ||||
| Class Arachnida | ||||
| Genera -Acariformes | ||||
| Familia Tetranychidae | ||||
| Tetranychus urticae Koch. | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Class Insecta | ||||
| Sub Class Apterigota | ||||
| Genera Podura | ||||
| Sminthurus viridis L. | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Sub Class Pterygota | ||||
| Genera Orthoptera | ||||
| Big Familia Tettigonioidae | ||||
| Familia Tettigoniidae | ||||
| Tettigonia caudata Charp. | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| viridissima L. | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Desticus albifrons Fabr. | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Phaneroptera falsata Poda. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Platycleis intermedia turanica Znr. | ▪▪▪▪ | ▪▪▪ | ■ | ♠ |
| Big Familia Grylloidae | ||||
| Familia Oecanthidae | ||||
| Oecanthus turanicus Uv. | ▪▪ | ▪▪ | ■ | ♠ |
| Familia Gryllidae | ||||
| Gryllus bimaculatus De.G. | ▪▪▪ | ▪▪▪▪ | ■ | ♠ ◙ |
| Tartarogryllus burdigalensis Latr. | ▪▪▪ | ▪▪▪▪ | ■ | ♠ ◙ |
| Melanogryllus desertus Pall. | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Pteronemobius heydeni P/Fisch. | ▪▪ | ▪▪▪ | ■ | ♠ ◙ |
| Familia Gryllotalpidae | ||||
| Gryllotalpa gryllotalpa L. | ▪▪ | ▪▪▪ | ■ | ♦ ◙ |
| Big Familia Acridoidae | ||||
| Familia Acrididae | ||||
| Calliptamus barbarus cephalotus F.-W. | ▪▪▪ | ▪ | ■ | ♠ |
| C.italicus italicus L. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| C.turanicus Serg.Tarb. | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Anacridium aegyptium L. | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Acrida oxycephala Pall. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Truxalis eximia Eichwald. | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Duronniella gracilis Uv. | ▪▪ | ▪▪▪ | ■ | ♠ |
| D.kalmyka Ad. | ▪ | ▪▪▪ | ■ | ♠ |
| Dociostaurus maracanus Thunb. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| D.tartarus Stshelk. | ▪▪ | ▪▪ | ■ | ♠ |
| D. kraussi nigoreniculatus Serg.Tarb. | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Eremipus persicus Uv. | ▪ | ▪▪ | ◘ | ♠ |
| Chorthippus (s. str.) dichrous Ev. | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Ch.turanicus Serg. Tarb. | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Epacromius tergestinus Charp. | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Ailopus oxianus Uv. | ▪▪ | ▪▪▪ | ◘ | ♠ |
| A.thallasinus Fabr. | ▪▪ | ▪▪▪▪ | ■ | ♠ |
| Locusta migratoria migratoria L. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Oedipoda miniata miniata Pall. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Sphingonotus satropes Sauss. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Spingoderes carinatus Sauss. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Oedaleus decorus Germ. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Genera Homoptera | ||||
| Sub Genera Auchenorrhyncha | ||||
| Familia Aphrophoridae | ||||
| Lepyronia coleoptrata L. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Philaenus spumarius L. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Familia Cicadellidae | ||||
| Hephathus unicolor Lindb. | ▪▪▪ | ▪▪▪ | ■ | ◙ |
| Austroagallia zachvatkini Vilb. | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Anaceratagallia venosa Fourc. | ▪▪ | ▪▪▪ | ■ | ♠ ◙ |
| A.aciculata Horv. | ▪▪ | ▪▪▪ | ■ | ◙ |
| A. kingradica Dub. | ▪▪ | ▪▪▪ | ■ | ◙ |
| A. laevis Rib. | ▪▪▪▪ | ▪▪▪▪ | ■ | ◙ |
| A. acuteangulata Zachv. | ▪▪ | ▪▪▪ | ■ | ♠ |
| A. alabugensis Dub. | ▪▪ | ▪▪▪ | ■ | ◙ |
| A. collicola Dub. | ▪▪ | ▪▪▪ | ■ | ◙ |
| A.turanica Dub. | ▪▪ | ▪▪▪ | ■ | ♠ |
| A.carsia Mit. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Batracomorhpus irroratus Lew. | ▪▪ | ▪▪▪ | ◘ | ♠ |
| Eupelix cuspidata F. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Aphrodes ferganensis Dub. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Cicadella viridis L. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Empoasca meridiana Zachv. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| E.minor Zachv. | ▪▪ | ▪▪▪▪ | ■ | ♠ |
| E.uzbekorum Zachv. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Kyboasca bipunctata Osh. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Chlorita aclydifera Dlab. | ▪▪ | ▪▪▪ | ◘ | ♠ |
| Asianidia asiatica Kush. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Pseudophlepsius binotatus Sign. | ▪▪ | ▪▪▪ | ■ | ♠ ◙ |
| Circulifer opacipensis Leth. | ▪▪ | ▪▪▪ | ■ | ♠ ◙ |
| C.haematoceps M.-R. | ▪▪ | ▪▪ | ■ | ♠ |
| C.tenellus Bak. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Macrosteles laevis Rib. | ▪▪ | ▪▪▪▪ | ■ | ♠ |
| M.quadripunctulatus Kbm. | ▪▪ | ▪▪▪ | ● | ♠ |
| Euscelidius mundus Hpt. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Euscelis lineolatus Brulle. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Eu. plebejus Fall. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Psanemotetix striatus L. | ▪▪▪ | ▪▪▪▪ | ■ | ♠ ◙ |
| P.dubovskyi Vilb. | ▪▪ | ▪▪▪ | ■ | ♠ ◙ |
| Familia Delphacidae | ||||
| Asiraca clavicornis Latr. | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Laodelphax striatellus Fall. | ▪▪ | ▪▪▪ | ■ | ♦ |
| Toya propinqua Fieb. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Ribautodelphax zeravshanicus Dub. | ▪▪ | ▪▪▪ | ■ | ♠ |
| Familia Dictyopharidae | ||||
| Distyophara europaea L. | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| D.longirostris Wlk. | ▪▪ | ▪▪▪ | ● | ♠ |
| Familia Сixiidae | ||||
| Pentastiridius pallens | ▪▪ | ▪▪▪▪ | ■ | ♠ |
| P.formicarius | ▪▪ | ▪▪▪ | ■ | ♠ |
| Reptalus rufocarinatus | ▪▪ | ▪▪▪ | ■ | ♠ |
| R.nigronervosus | ▪▪ | ▪▪▪▪ | ■ | ♠ |
| Hyalesthes obsoletus | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Familia Tettigometridae | ||||
| Tettigometra varia | ▪▪ | ▪▪ | ◘ | ♠ |
| T.vitellina | ▪▪ | ▪▪▪ | ■ | ♠ |
| Familia Issidae | ||||
| Scorlupaster asiaticus | ▪▪ | ▪▪ | ■ | ♠ |
| Brachyprosopa bicornis | ▪ | ▪▪ | ■ | ♠ |
| B.umnovi | ▪▪ | ▪▪▪ | ■ | ♠ |
| Sub Genera Aphidinea | ||||
| Familia Callaphididae | ||||
| Therioaphis trifolii | ▪▪ | ▪▪▪ | ■ | ♠ |
| Familia Aphididae | ||||
| Triba Aphidini | ||||
| Sub Triba Aphidina | ||||
| Aphis craccivora | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| SubTriba Macrosiphina | ||||
| Acyrhosiphon pisum | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Genera Hemiptera | ||||
| Familia Pentatomidae | ||||
| Antheminia lunulata | ▪▪ | ▪▪▪ | ■ | ♠ |
| A.aliena | ▪▪ | ▪▪▪ | ■ | ♠ |
| Brachynema germarii | ▪▪ | ▪▪▪ | ■ | ♠ |
| Holcostethus peltatus | ▪▪▪ | ▪▪▪▪ | ◘ | ♠◘ |
| Dolycoris penicillatus | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Codophila varia | ▪▪ | ▪▪▪▪ | ■ | ♠ |
| Familia Coreidae | ||||
| Camptopus lateralis | ▪▪▪ | ▪▪▪ | ● | ◘ |
| Coroimeris vitticollis | ▪▪ | ▪▪▪ | ● | ◘ |
| Familia Miridae | ||||
| Adelphocoris jakovlevi | ▪▪▪ | ▪▪▪ | ◘ | ♥ |
| A.lineolatus | ▪▪▪▪ | ▪▪▪▪ | ■ | ♥ |
| Brachycoleus decolor | ▪▪ | ▪▪▪ | ■ | ♥ |
| Camptobrochis punctulatus | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Campylomma verbasci | ▪▪ | ▪▪▪ | ■ | ♠ |
| Lygus gemellatus | ▪▪▪▪ | ▪▪▪▪ | ■ | ♥◘ |
| L.pratensis | ▪▪▪▪ | ▪▪▪▪ | ■ | ♥◘ |
| L.rugulipennis | ▪▪ | ▪▪▪ | ■ | ♥◘ |
| Plagiognathus bipunctatus | ▪▪ | ▪▪▪ | ● | ♥ |
| Poeciloscytus cognatus | ▪▪▪▪ | ▪▪▪▪ | ■ | ♥ |
| P.vulneratus | ▪▪▪ | ▪▪▪▪ | ■ | ♥ |
| Trigonotylus ruficornis | ▪▪ | ▪▪▪▪ | ■ | ♥ |
| Genera Thysanoptera | ||||
| Sub Genera Terebrantia | ||||
| Familia Thripidae | ||||
| Frankliniella intonza | ▪▪▪ | ▪▪▪ | ■ | ♥ |
| F.pallida | ▪▪▪ | ▪▪▪▪ | ■ | ♥ |
| Kakothrips robustus | ▪▪▪ | ▪▪▪ | ● | ♠ |
| Odontothrips confusus | ▪▪ | ▪▪▪ | ● | ♥ |
| O.phaleratus | ▪▪▪ | ▪▪▪ | ● | ♥ |
| O.loti | ▪ | ▪▪▪ | ■ | ♥ |
| Thrips tabaci | ▪▪▪▪ | ▪▪▪▪ | ■ | ♥ |
| Subgenera Tubulifera | ||||
| Familia Phloeothripidae | ||||
| Haplothrips reuteri | ▪▪ | ▪▪▪ | ■ | ♥ |
| Genera Coleoptera | ||||
| Familia Carabidae | ||||
| Аnisodactylus pseudoaeneus | ▪▪▪▪ | ▪▪▪ | ◘ | ♠ |
| Amara similata | ▪▪▪ | ▪▪▪▪ | ■ | ♥ |
| Ditomus semicylindricus | ▪▪▪ | ▪ | ■ | ♠ |
| Ophonus calceatus | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| O.rufipes | ▪▪▪▪ | ▪▪▪ | ■ | ♠ |
| Zabrus morio | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Familia Silphidae | ||||
| Aclypaea turkestanica | ▪▪ | ▪▪▪ | ◘ | ♠ |
| Silpha tristis subsp. Costata | ▪▪ | ▪▪▪ | ◘ | ♠ |
| Familia Scarabaeidae | ||||
| Lethrus appendiculatus | ▪▪▪ | ▪▪▪ | ■ | ◙ |
| L.costatus | ▪▪▪ | ▪▪▪ | ■ | ◙ |
| L.rosmarus | ▪▪ | ▪▪▪ | ■ | ◙ |
| L.microbuccus | ▪▪▪ | ■ | ◙ | |
| L.superbus | ▪▪▪ | ▪▪▪▪ | ■ | ◙ |
| L.pygmaeus | ▪▪▪ | ▪▪▪▪ | ■ | ◙ |
| Small Familia Rutelinae | ||||
| Cyriopertha glabra | ▪▪▪ | ■ | ♦ | |
| Small Familia Melolonthinae | ||||
| Cryphaeobius brunneus | ▪▪▪ | ▪▪▪ | ■ | ♦ |
| Lasiexis dilaticollis | ▪▪ | ▪▪▪ | ■ | ♦ |
| Panotrogus myschenkovi | ▪▪▪ | ■ | ♦ | |
| Xanthotrogus fortis | ▪▪▪ | ▪▪▪ | ■ | ♦ |
| Small Familia Cetoniinae | ||||
| Cetonia aurata | ▪▪▪ | ▪▪▪▪ | ■ | ♥ |
| Epicometis turanica | ▪▪▪ | ▪▪▪▪ | ■ | ◙ |
| Oxythyrea cinctella | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Familia Melyridae | ||||
| Malachius aeneus | ▪▪ | ▪▪▪ | ■ | ♠ |
| M.ambiguus | ▪ | ▪▪ | ◘ | ♠♥ |
| Familia Elateridae | ||||
| Adelocera funebris | ▪▪ | ▪▪▪ | ■ | ♦ |
| Agriotes caspicus | ▪▪▪ | ▪▪▪ | ■ | ♦ |
| A.meticulosus | ▪▪▪ | ▪▪▪ | ■ | ♦ |
| Familia Buprestidae | ||||
| Julodis variolaris | ▪▪ | ▪▪▪ | ■ | ♦ |
| Sphenoptera (s.str.) laticeps | ▪▪▪ | ▪▪▪ | ◘ | ♦ |
| S. (s.str.) mohtana | ▪▪▪▪ | ▪▪ | ◘ | ♦ |
| Familia Nitidulidae | ||||
| Melygethes planisculus | ▪▪ | ▪▪▪ | ◘ | ♥ |
| Familia Mordellidae | ||||
| Mordellistena pumilla | ▪▪ | ▪▪ | ◘ | ♠ |
| Familia Tenebrionidae | ||||
| Gonocephalum rusticum | ▪▪▪ | ▪▪▪ | ■ | ◙ |
| Tenebrio obscurus | ▪▪▪ | ▪▪▪ | ■ | ◙ |
| Familia Meloidae | ||||
| Epicauta erythrocephala | ▪▪▪ | ▪▪ | ■ | ♥ |
| Mylabris bigutteta | ▪▪▪ | ▪▪▪▪ | ■ | ♥ |
| M.calida | ▪▪ | ▪▪▪ | ■ | ♥ |
| M.crocata | ▪▪ | ▪▪ | ■ | ♥ |
| M.monnerheimi | ▪▪ | ▪▪ | ■ | ♥ |
| M.scabiosae | ▪▪▪ | ▪▪▪▪ | ■ | ♥ |
| Familia Cerabycidae | ||||
| Agapanthia violacea | ▪▪ | ▪▪ | ■ | ♠ |
| Familia Chrysomelidae | ||||
| Small Familia Clytrinae | ||||
| Labidostomis metallica centrisculpta | ▪▪▪▪ | ▪▪▪ | ■ | ♠ |
| Familia Сurculionidae | ||||
| Apion facetum | ▪▪ | ■ | ♠♥ | |
| A.filirostre | ▪▪▪ | ▪▪▪ | ◘ | ♠ |
| A.flavofemaratum | ▪▪ | ● | ♦ | |
| A.seniculus | ▪▪ | ▪▪▪ | ● | ♦ |
| A.tenue | ▪▪▪ | ▪▪▪ | ● | ♠ |
| Eusomus ovulum | ▪▪ | ▪▪▪ | ■ | ♠ |
| Myllocerus benignus | ▪▪ | ▪▪▪ | ■ | ♠ |
| Phytonomus variabilis | ▪▪▪▪ | ▪ | ◘ | ♠ |
| Sitona callosus | ▪▪ | ▪▪▪ | ● | ♠ ◙ |
| S.costipennis | ▪▪ | ▪▪▪ | ◘ | ◙ |
| S.crinitus | ▪▪ | ▪▪▪ | ■ | ◙ |
| S.cylindricollis | ▪▪▪▪ | ▪▪▪ | ● | ◙ |
| S.flavescens | ▪▪▪ | ▪▪▪ | ● | ◙ |
| S.fronto | ▪▪▪ | ◘ | ||
| S.humeralis | ▪▪▪ | ▪▪▪ | ● | ♦ |
| S.inops | ▪▪▪ | ▪▪ | ● | ♠ |
| S.lineellus | ▪▪▪ | ▪▪ | ● | ♠ |
| S.longulus | ▪▪▪ | ● | ♠♦ | |
| S.sulcifrons | ▪▪ | ▪ | ● | ♠♦ |
| Tychius aureolus ssp. femoralis | ▪▪▪ | ▪▪▪ | ● | ◘ |
| T.flavus | ▪▪▪▪ | ◘ | ◘ | |
| T.junceus | ▪▪ | ▪▪▪ | ● | ◘ |
| Genera Lepidoptera | ||||
| Familia Lithocolletidae | ||||
| Lethocolletius insignitella | ▪▪▪ | ▪▪ | ■ | ♠ |
| Familia Tortricidae | ||||
| Clepsis strigona | ▪▪▪ | ▪▪ | ■ | ♠ |
| Familia Crambidae | ||||
| Nyctegretis achatinella | ▪▪ | ● | ♠ | |
| Familia Noctuidae | ||||
| Agrotis segetum | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ ◙ |
| A. exclamationis | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| A.ipsilon | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Xestia c-nigrum | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Discestra trifolli | ▪▪▪ | ▪▪▪ | ■ | ♠♥ |
| Mamestra aleracea | ▪▪ | ▪▪▪ | ■ | ♠ |
| Mythimma unipuncta | ▪▪ | ▪▪▪ | ■ | ♠ |
| Spodioptera exigua | ▪▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Heliothis viriplaca | ▪▪▪▪ | ▪▪▪ | ■ | ♠ |
| H. peltigera | ▪▪ | ▪▪▪ | ■ | ♠ |
| Helicoverpa armigera | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Syngrapha circumflexa | ▪▪▪ | ▪▪▪▪ | ■ | ♠ |
| Autographa gamma | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Familia Pieridae | ||||
| Colias erate | ▪▪ | ▪▪▪ | ● | ♠ |
| Familia Lycaenidae | ||||
| Palyommatus icarus | ▪▪▪ | ▪▪▪ | ■ | ♠ |
| Familia Geometridae | ||||
| Tephrina avenaceria | ▪▪▪ | ▪▪▪ | ■ | ♠♥ |
| Anaitis (Aplocera) plagiata | ▪▪ | ■ | ♠♥ | |
| Genera Hymenoptera | ||||
| Sub Genera Apocrita | ||||
| Big Familia Chalcidoidae | ||||
| Familia Eurytomidae | ||||
| Bruchophagus roddi | ▪▪▪▪ | ● | ◘ | |
| Big Familia Apoidae | ||||
| Familia Megachilidae | ||||
| Megachile pacifica | ▪▪▪ | ■ | ♠♥ | |
| Genera Diptera | ||||
| Familia Cecidomyiidae | ||||
| Contarinia loti | ▪▪▪ | ● | ♥ | |
| C.medicaginis | ▪▪▪ | ◘ | ♥ | |
| Dasyneura ignorata | ▪▪▪ | ◘ | ♠ | |
Table 1. Composition of species and nutritional characteristics of phytophagous alfalfa agrobiocenosis.
Conclusion
The study of the formation of alfalfa entomofauna showed that it directly related the formation of harmful entomofauna in annual alfalfa to the amount of food. Because of the instability of the plant phytomass, all-consuming pests predominate in the crop. These species feed on weeds that growing in the field and in the alfalfa. Later, due to the formation of plant (alfalfa) cover, oligophages displace all species. This is because the old alfalfa is the primary source for the transition of monophages to the crop.
We observe the process of formation of beneficial entomofauna of alfalfa due to transferring entomophagous plants from weeds around the crop. However, the process of formation of entomofauna, including beneficial fauna, can be influenced by agrotechnical measures applied to the cultivation of alfalfa and cotton rotation.
References
Ellington, J., Southward, M., Carrillo, T. (1997). Association Among Cotton Arthropods. Environmental Entomology, 26:1004–1008.
Hoch, G., Schopf, A. (2001). Effects of Glyptapanteles liparidis (Hym.: Braconidae) parasitism, polydnavirus, and venom on development of microsporidia-infected and uninfected Lymantria dispar (Lep.; Lymantriidae) larvae. Journal Invertebr Pathology, 77:37-43.
Khamraev, A.Sh. (1999). Seasonal distribution of field bugs in agrobiocenoses. Plant Protection and Quarantine, 9:31.
Khamraev, A.Sh., Abdullaeva, D.R. (2000). Composition of entomophages of alfalfa, regulation of the state of abundance of dominant harmful species. Uzbek Biological Journal, Tashkent: Science, 3:45-48.
Khamraev, A.Sh., Abdullaeva, D.R. (2001). Semi-hard-breasted alfalfa agrobiocenosis of the north-west and north-east of Uzbekistan. Proceedings of Universities. Chemical and Biological Sciences, Tashkent, 2:23-25.
Khamrayev, A.Sh., Abdullayeva, D.R. (2005). Phytophagous alfalfa agrobiocenosis. Journal of Biology of Uzbekistan, Tashkent: Science, 4:57-61.
Author Info
A.D. Rixsikhodjaevna1*, R.A. Yusupovich2 and H.P. Bobakulovna12Karshi State University, Uzbekistan
Citation: Rixsikhodjaevna, A.D., Yusupovich, R.A., Bobakulovna, H.P. (2021). A formation of alfalfa entomofauna. Ukrainian Journal of Ecology. 11:1-8.
Received: 19-Oct-2021 Accepted: 03-Dec-2021 Published: 30-Dec-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
