Research Article - (2025) Volume 15, Issue 3
Carbon stock estimation for coarse woody debris in a northern Republic of Congoâs forest
Romeo Ekoungoulou1*, Bienfaite Jariya Bamissamou1, Saint Fedriche Ndzaï1, Fousseni Folega2, Chrisveil de Ben-Mack Mbouilou1, Jafrel Lo�ck Bikouta1 and Felix Koubouana1Abstract
Coarse Woody Debris (CWD) is an important carbon store in tropical forest ecosystems. The study carried out in the Ipendja forest in the Likouala Department, Republic of Congo, aimed to improve knowledge of forest ecosystem functioning. The research was carried out in six rectangular plots, each 5000 m2 (i.e. 200m × 25m) with a total of 149 CWD sampled. All individuals with diameter ≥10 cm were inventoried for coarse woody debris carbon stock estimation. Standing dead trees (snags) and fallen dead trees and branches (logs) were recorded. The results of this study revealed that the average stock of coarse woody debris was 16.55 t ha-1, with a stock of 33.09 t C ha-1 in the logs plots, and that of dry mass in the snags was 2.99 t ha-1, with a storage rate of 1.49 t C ha-1. This study has shown that windthrow plays a key role in the production of large coarse woody debris. Carbon stocks in this study area revealed that fallen dead trees and branches on the ground has a higher carbon content than standing dead trees.Keywords
Carbon stock, Snags, Logs, Coarse woody debris, Ipendja forest.
Introduction
For almost two centuries, planet Earth has been experiencing remarkable changes in the chemical composition of its atmosphere. These changes have resulted in a significant increase in the atmospheric concentration of various greenhouse gases, notably CO2, the main product of the combustion of organic matter (Ifo AS. 2010). There are two main reasons for this increase: the use of fossil fuels and changes in land use (Ifo AS. 2010 and Ekoungoulou R. et al., 2021). It has become clear that the increase in Greenhouse Gas (GHG) content in the atmosphere and the resulting climate change will have major effects in the 21st century (Zoghaib R. 2021). Temperatures have already risen by 1°C, and although scenarios are still uncertain, by 2100 the average global temperature is expected to rise by 1.5 to 6°C (Moorhead et al., 2016) if no action is taken to reduce sources of CO2 emissions.
The Congo Basin forest massif constitutes the second largest reserve of dense rainforests in the world (70% of African reserves) after the Amazon forest massif (Ifo AS. 2010; Baker TB. et al., 2011 and Ekoungoulou R. et al., 2017). Several authors have shown that, forests in general more particularly tropical forests remain the only means of combating climate change (Nasi R. et al., 2009; Lewis SL. et al., 2013 and Ifo AS. et al., 2018). However, the functioning of the tropical rainforests of the Congo Basin is still poorly understood, although their role in climate mitigation has been reported for several decades (Ekoungoulou R. et al., 2018 and Ifo AS. et al., 2018). Tropical forests contain 40-50% of terrestrial carbon and play a major role in the global carbon cycle (Pan Y. et al., 2011).
The loss of forest cover resulting from deforestation and forest degradation contributes around 10-15% of annual global greenhouse gas emissions (Van der Werf GR. et al., 2009 and Ekoungoulou R. 2014). The United Nations Framework Convention on Climate Change has examined the possibility of reducing these emissions through the international REDD+ (Reducing Emissions from Deforestation and forest Degradation, including Sustainable Forest Management, Biodiversity Conservation and Carbon stock enhancement in developping countries) initiative (Ekoungoulou R. et al., 2015). The role of tropical forests in climate regulation and biodiversity conservation is well established (Pascal JP. 2003). In forest ecosystems, carbon is stored in living biomass (aboveground and belowground biomass), coarse woody debris, soil organic matter and litter (Penman J. et al., 2003), containing more than 3/4 of the forest's carbon.
Coarse Woody Debris (CWD) or Coarse Woody Habitat (CWH) refers to fallen dead trees and the remains of larges branches on the ground in forests. Coarse woody debris is one of the compartments of forest ecosystems, and is involved in the structure and functioning of forest ecosystems (Bocko YE. et al., 2017 and Ekoungoulou R. et al., 2018). They constitute an important carbon reservoir in forest ecosystems both as a source and sink of CO2 for the atmosphere (Baker TR. et al., 2007). Coarse woody debris has been found to account for around 11% of the total carbon stock in African tropical forest ecosystems, and to contain between 0 and 48 t C ha-1 in tropical forests, varying according to forest type (Ndzaï SF. 2022).
With over 23,517,000 ha, or 69.8% of the national territory (MEF, 2021). Congolese forests play a very important role in combating climate change in several sectors. However, several studies have been conducted in various carbon pools, but that of the coarse woody debris compartment seems to be neglected (Ifo AS. et al., 2018). Some studies undertaken in Congolese forests on dead wood are: Ifo AS. (2010) in the Plateaux Teke; Bocko YE. et al., (2017) in Likouala; Ifo AS. et al., (2018) in Likouala; Ekoungoulou R. et al., (2018) in Likouala and Ndzaï SF (2022) also in Likouala. In fact, these studies did not cover the entire Likouala department, which has a large forest area. A specific inventory of the coarse woody debris compartment of Likouala forests in general, and those of the Ipendja FMU in particular, is therefore necessary to estimate the total carbon stock contained in this compartment. It is also important to know that the large woody debris compartment is an indicator of the state of equilibrium of a forest ecosystem. In the current context, this should increase our knowledge of the functioning of Congo Basin forests in general, and Likouala forests in particular, and improve our estimates of the percentage of CO2 captured by these forests. In fact, carbon inputs to the soil in ecosystems depend on standing biomass, tree residues of all kinds, organ lifespan (turnover), the specific composition of the stand and its density, and the edaphic and climatic characteristics of the site (Bocko YE et al., 2017). Coarse woody debris can have significant effects on flow structure (Abbe TB. et al., 1996), sediment transport (Wohl E. 2011), fluvial form organization (Montgomery DR. et al., 1995) and fluvial style (Sear DA. et al., 2010). It is in this context that, this study was carried out to understand the functioning of forest ecosystems in the Congo in general and the Likouala department in particular. The aim was to improve knowledge of the carbon stock in large woody debris, and to ensure sustainable and rational management of forest ecosystems to combat climate change.
This study had three objectives: 1- to estimate carbon stock by deadwood type; 2- to estimate deadwood carbon stock by decomposition class; 3- to determine deadwood carbon stock by diameter class.
Materials and Methods
Study area
The study area is located in the northern Republic of Congo, precisely in the Forest management unit (UFA) Ipendja, which is situated in the Dongou district from Likouala Department in the Republic of Congo. The Ipendja forest management unit is part of Zone II (Ibenga-Motaba) with an estimated area of 461,296 hectares (Ekoungoulou R. et al., 2018 and STC, 2022). This Department extends over 230 km from east to west and around 550 km from north to south. It is bordered: to the north by the Central African Republic, to the south by the Central Basin Department, to the east by the Oubangui River, which separates it from the Democratic Republic of Congo, and to the west by the Sangha Department (Ifo AS. et al., 2018) (Fig. 1).
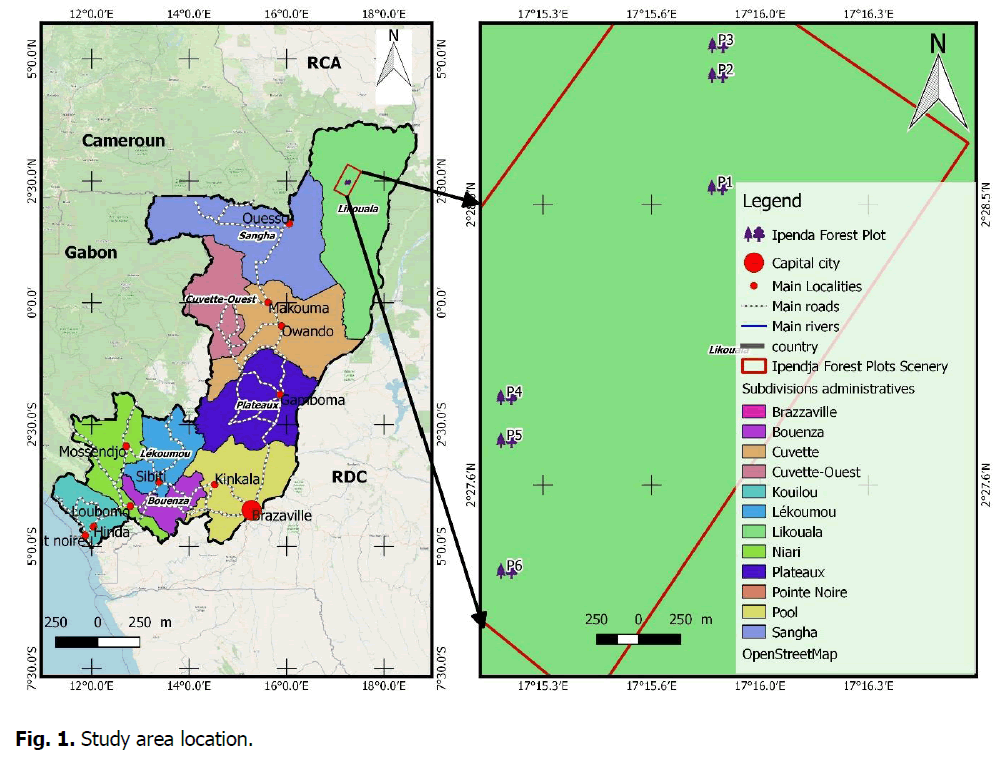
Figure 1. Study area location.
Climate: The study area has a humid tropical equatorial climate from the Guinean forest type. The average temperature in Likouala Department is 25 °C, while average rainfall ranges from 1,600 mm to 1,800 mm, with interannual variability of 10 to 15%. There is a 40-day dry season from December to January; an intra-rainy dip in July, with average annual temperatures of 25-26 °C (Fig. 2) and an amplitude of 1 to 2 °C (ANAC, 2022); and relative air humidity of 84 to 86% all year round (ANAC Congo data for averages from 2010 to 2021).
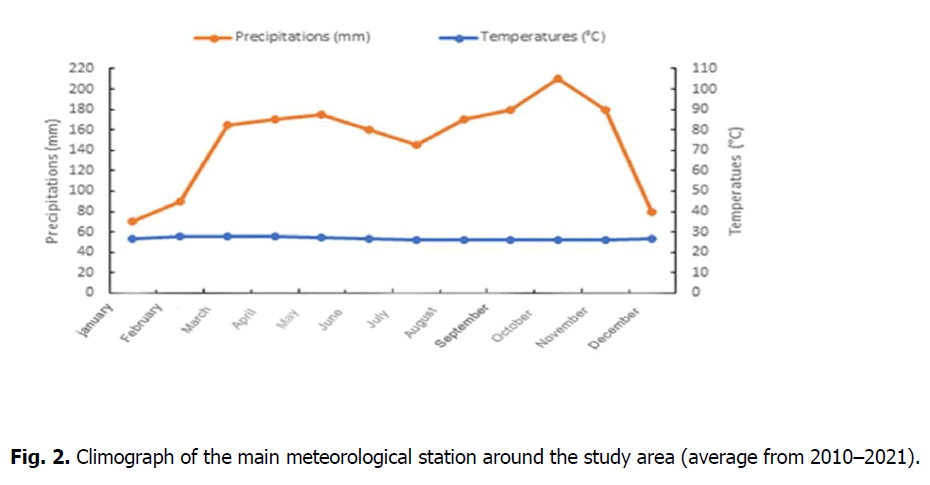
Figure 2. Climograph of the main meteorological station around the study area (average from 2010–2021).
Data collection: Plots were laid out according to the configuration of the area and compass directions (north-south and east-west), so as to obtain rectangular plots of 0.5ha, i.e. 25 m × 200 m or 5000 m2. Each plot was subdivided into plots of four. In all, six plots were set up with a total area of 5000 m2 in this zone.
Within each plot, dead wood samples were collected taking into account the two types of wood, namely: Large woody debris or dead wood lying on the ground or "Logs" (Pedlar JH. et al., 2002), large woody debris is defined in this study as all woody debris lying, in direct contact or not with the ground and having a diameter ≥ 10 cm (Ekoungoulou R. et al., 2018). "Snags" are standing woody debris with a diameter ≥ 10 cm (Ekoungoulou et al., 2018b). The practical method for categorizing the decomposition of dead trees still standing is explained as follows: Category 1: Tree with branches and twigs resembling a living tree (except for foliage); Category 2: Twigless tree with large and small branches; Category 3: Trees with large branches; Category 4: Trunk without branches.
Method for identifying decomposition classes of woody plant debris
The decomposition classes of plant woody debris were studied during the census of Logs and Snags. The physical state of the woody debris is assessed according to the resistance of the wood to the penetration of a metal (in our study, the machete) into the body of the woody plant debris (Clark DF. et al., 1998; Laiho R. 1999). For this study, we defined four decomposition classes following the protocol defined by Ifo AS. et al., (2018): Class I: More than 75% of the wood is still intact, and much of the bark is still intact; metal does not penetrate; Class II: 25-50% of the wood is beginning to soften, and the bark only partly covers the dead wood; Class III: 75% of tree trunk decomposed, bark completely degraded; Class IV: more than 75% of the wood is decomposed, and metal penetrates very easily.
Method for identifying the diameter class for coarse woody debris
For the diameter class identification method, we only measured dead wood with a diameter of 10 cm or more. Dead wood with a diameter of less than 10 cm was not recorded.
Data analysis
Logs volume calculation
To calculate the volume of logs or dead wood, we used model proposed (Equation 1) by Warren W. and Olsen PF. (1964):
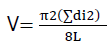 (Equation 1)
(Equation 1)
V= volume of coarse woody debris (m3. ha-1); di, is the diameter of each woody debris sampled (m); L= 100. Conversion of the results obtained from volume to mass is obtained by setting the wood density value at 0.5 Kg MS.m-3.
Snags volume calculation
To calculate the volume of standing dead trees, the following model developed (Equation 2) by Mund M. (2004) was used:
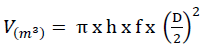 (Equation 2)
(Equation 2)
V is the volume of standing dead trees per unit area (m3); π is 3.14; D is the diameter of standing dead trees (cm), f is the form factor with a value of 0.627; h is the height of standing dead trees (m).
Statistical analysis
To process the data, tables and graphs were drawn up using Microsoft Excel version 2019, into which we entered all the information collected in the field. Data collected was subjected to analysis of variance (ANOVA) to determine if differences between each variable is statisticaly significative. The statistical analysis was performed using SPSS version 26.2. software and PAST v 3.05 including standard statistical tests at p<0.05.
Results
Composition of coarse woody debris
The coarse woody debris study carried out in the Likouala Department, more specifically in the Ipendja FMU, identified a total of 149 samples of deadwood with a DBH 10 cm in six rectangular plots measuring 200 m × 25 m, i.e. 5,000 m2 or 0.5 ha in area. They are distributed as follows: 114 Logs and 35 Snags.
Table 1 shows that the highest number of Snags has been founded in plot 1 and the lowest in plot 6, while the highest numbers of logs have been founded in plot 2 and plot 4, and the lowest in plot 3.
| Plots | Number of Snags | Number of Logs | Total |
|---|---|---|---|
| P1 | 10 | 14 | 24 |
| P2 | 9 | 23 | 32 |
| P3 | 9 | 12 | 21 |
| P4 | 4 | 23 | 27 |
| P5 | 2 | 22 | 24 |
| P6 | 1 | 20 | 21 |
| Total | 35 | 114 | 149 |
Table 1. Number of Snags and logs in study area.
In terms of dead wood content per study plot, the highest percentage was recorded in plot 2 at 22%, followed by plot 4 at 18%, then plots 1 and 5 at 16% and finally plots 3 and 6 at 14% (Fig. 3a). With regard to Snags, the highest percentage of carbon stock was found in plot 5 with 43% or 1.49 t C ha-1, followed by 25% or 0.88 t C ha-1 in plot 2 with 14% or 0.50 t C ha-1 in plot 3 with 10% or 0.35 t C ha-1 in plot 1, then 7% or 0.24 t C ha-1 in plot 6 and finally the lowest 1% or 0.04 t C ha-1 in plot 4 (Fig. 3b). Fig. 3c shows the carbon stock of standing deadwood (Snags). Values vary from plot to plot (Fig. 3c).
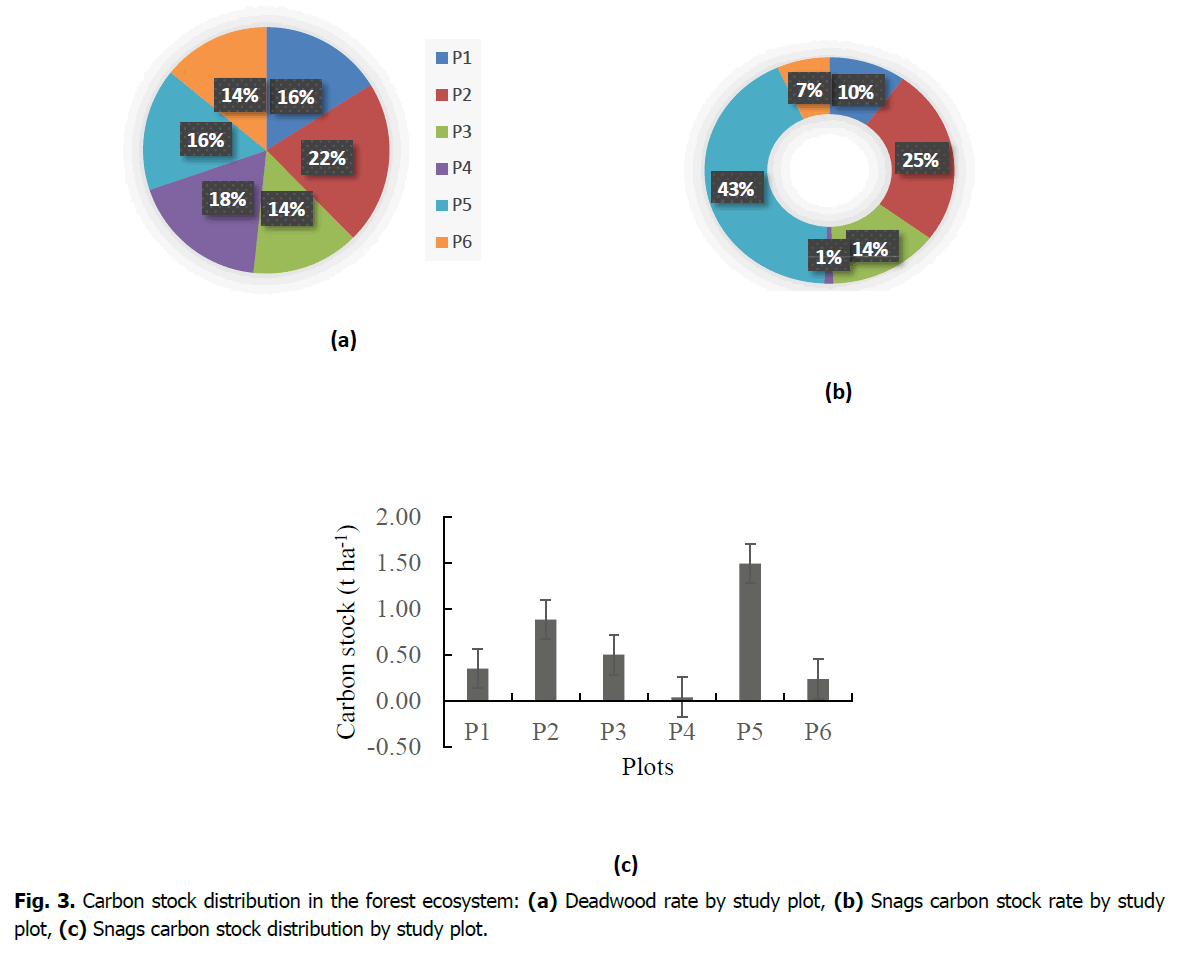
Figure 3. Carbon stock distribution in the forest ecosystem: (a) Deadwood rate by study plot, (b) Snags carbon stock rate by study plot, (c) Snags carbon stock distribution by study plot.
Decomposition class for coarse woody debris
Deadwood decomposes very slowly. We have identified several classes of different types of dead wood. Table 2 shows the variation in decomposition of standing dead wood in relation to the plots. This table shows that the most represented class is class II with 7.28 t C ha-1 in plot 3 and 5.23 t C ha-1 in plot 5, followed by class I with 1.35 t C ha-1 in plot 2 and 1.24 t C ha-1 in plot 1. The least represented class is class IV, with 0.03 t C ha-1 in plot 4.
| Decomposition Class | Plots | |||||
|---|---|---|---|---|---|---|
| P1 (t C ha1) |
P2 (t C ha-1) |
P3 (t C ha-1) |
P4 (t C ha-1) |
P5 (t C ha-1) |
P6 (t C ha-1) |
|
| Class I | 1.24 | 1.35 | 0.39 | 0 | 0.17 | 0 |
| Class II | 0 | 0.63 | 7.28 | 0.08 | 5.23 | 0 |
| Class III | 0 | 0 | 0 | 0 | 0 | 0 |
| Class IV | 1.1 | 0.51 | 0.26 | 0.03 | 0 | 0.24 |
Table 2. Decomposition class for snags.
Table 3 shows that the first class is the most represented with 90.48 t C ha-1 in plot 6, followed by class IV with 49.8 t C ha-1 in plot 2. The least represented class is class IV with 1.77 t C ha-1 in plot 5.
| Decomposition Class | Plots | |||||
|---|---|---|---|---|---|---|
| P1 (t C ha-1) |
P2 (t C ha-1) |
P3 (t C ha-1) |
P4 (t C ha-1) |
P5 (t C ha-1) |
P6 (t C ha-1) |
|
| Class I | 36.81 | 37.36 | 2.62 | 34.89 | 8.94 | 90.48 |
| Class II | 10.22 | 27.66 | 3.13 | 31.98 | 55.77 | 19.15 |
| Class III | 0 | 31.98 | 7.10 | 28.97 | 8.8 | 4.92 |
| Class IV | 12.10 | 49.8 | 28.86 | 27.48 | 1.77 | 37.74 |
Table 3. Decomposition class for logs.
Diameter class for coarse woody debris
Tables 4 and 5 shows how these values vary from plot to plot. Table 4 shows the diameter classes of standing deadwood or Snags. Table 5, on the other hand, shows the variable values for the diameter classes of large woody debris lying on the ground, or Logs.
| Diameter Class | Plots | |||||
|---|---|---|---|---|---|---|
| P1 (t C ha-1) |
P2 (t C ha-1) |
P3 (t C ha-1) |
P4 (t C ha-1) |
P5 (t C ha-1) |
P6 (t C ha-1) |
|
| Class I | 0.03 | 0.06 | 0.02 | 0 | 0 | 0 |
| Class II | 0.17 | 0.08 | 0.12 | 0.02 | 0.17 | 0 |
| Class III | 0 | 0 | 0 | 0.07 | 0 | 0 |
| Class IV | 2.17 | 0 | 0.49 | 0 | 0 | 0 |
| Class V | 0 | 1.20 | 0 | 0 | 0 | 0 |
| Class VI | 0 | 1.19 | 0 | 0 | 0 | 0 |
| Class VII | 0 | 0.42 | 0 | 0 | 0 | 0 |
| Class VIII | 0 | 0 | 0 | 0 | 5.23 | 0 |
| Class IX | 9.07 | 2.18 | 0 | 0 | 0 | 0.24 |
| Class X | 0 | 0 | 0 | 0 | 0 | 0 |
| Class XI | 0 | 0 | 0 | 0 | 0 | 0 |
| Class XII | 0 | 0 | 29.06 | 0 | 0 | 0 |
Table 4. Diameter class of standing coarse woody debris.
| Diameter class | Plots | |||||
|---|---|---|---|---|---|---|
| P1 (t C ha-1) |
P2 (t C ha-1) |
P3 (t C ha-1) |
P4 (t C ha-1) |
P5 (t C ha-1) |
P6 (t C ha-1) |
|
| Class I | 3.57 | 3.11 | 2.94 | 3.99 | 2.78 | 1.78 |
| Class II | 7.32 | 8.02 | 7.1 | 6.58 | 6.86 | 6.79 |
| Class III | 16.87 | 13.38 | 13.09 | 13.39 | 13.02 | 15.10 |
| Class IV | 26.13 | 25.75 | 0 | 19.72 | 19.72 | 19.72 |
| Class V | 30.81 | 37.28 | 0 | 35.09 | 0 | 0 |
| Class VI | 51.02 | 49.72 | 0 | 44.37 | 44.37 | 44.37 |
| Class VII | 0 | 60.39 | 60.39 | 60.39 | 0 | 60.39 |
| Class VIII | 0 | 78.88 | 0 | 80.21 | 0 | 78.88 |
| Class IX | 0 | 99.83 | 0 | 0 | 0 | 0 |
| Class X | 0 | 123.25 | 0 | 0 | 0 | 0 |
| Class XI | 0 | 0 | 0 | 0 | 149.13 | 0 |
| Class XII | 0 | 0 | 0 | 0 | 177.47 | 0 |
Table 5: Distribution of logs by diameter class according to study plots.
In the six plots studied, the highest rate was obtained in plot 2 with 27% or 28.49 t/ha, followed by 23% or 24.33 t/ha in plot 4, 19% or 19.87 in plot 6, 16% or 16.55 in plot 1, then 9% or 9.59 in plot 5 and finally the lowest 6% or 6.75 in plot 3 (Fig. 4).
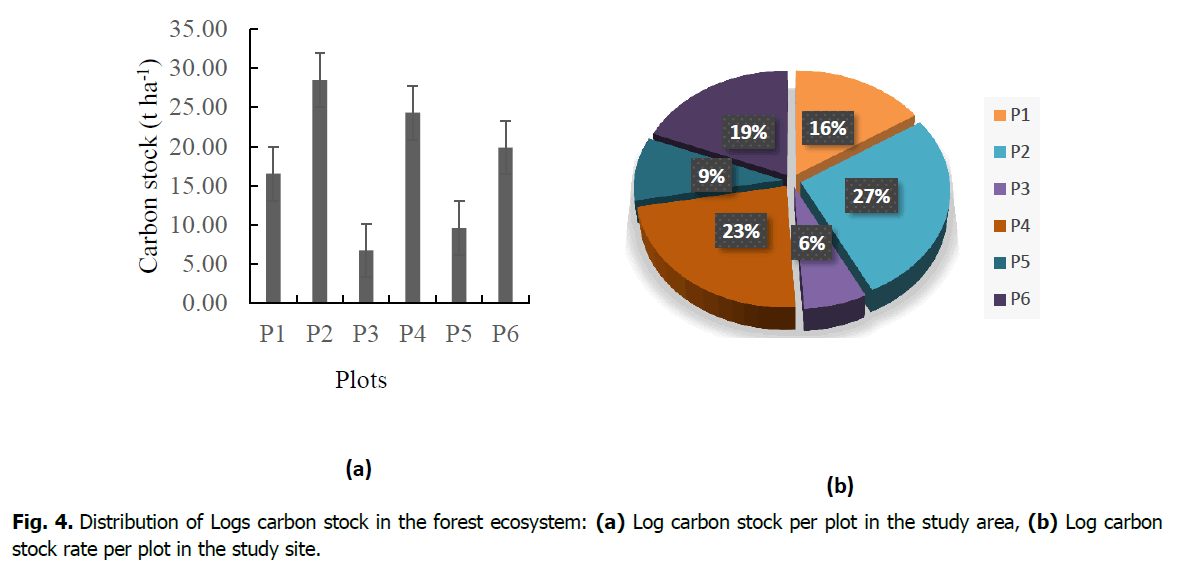
Figure 4. Distribution of Logs carbon stock in the forest ecosystem: (a) Log carbon stock per plot in the study area, (b) Log carbon stock rate per plot in the study site.
However, following the results on the carbon stock of each type of dead wood, we carried out Student's t-test with independent samples. But first, the normality of the data was checked by performing a normality test, the results of which show that for the carbon of large woody debris lying on the ground, ddl꞊6 and P-value꞊0.841; for the carbon of large woody debris standing on the ground, ddl꞊6 and P-value꞊0.473. After checking the normality of the data, we performed Student's t-test with independent samples. This test revealed the existence of significant differences (value꞊0.001) between carbon stored in lying deadwood and carbon stored in standing deadwood.
Discussion
The study on carbon stock estimation of dead wood in the Ipendja Forest Management Unit (FMU), Likouala Department obtained one hundred and forty-nine (149) samples with diameters ≥ 10 cm, including 35 Snags and 114 Logs. Our results are superior to those of Ekoungoulou R. et al., (2018) on the study of the carbon stock of coarse woody debris in Central Africa, who obtained 135 samples with diameters greater than or equal to 10 cm in the two sites: that of Mokelimwaeki (site 1, n= 57) and Sombo (site 2, n= 78). The high number of our results can be justified by a number of factors, such as strong winds, forest ageing, the fall of one tree dragging down the others, and bark beetles.
Type of coarse woody debris
Stocks of coarse woody debris obtained during our study show great spatial variability between types of dead wood. Results indicate that plot 5 has the highest carbon stock of the snags (1.49 t C ha-1). This predominance is certainly due to the lack of light reaching the ground, which causes the mortality of certain heliophilous species. In fact, we observed that the canopy was closed. In the case of dead wood lying on the ground, we observed more carbon storage than standing dead wood. The study shows a predominance of carbon stock in plots 2 and 4, with 28.49 t C ha-1 and 24.33 t C ha-1 respectively. This predominance is due to the fact that we observed several dead trees lying on the ground, caused by natural factors - the recurrent phenomenon of windfall and the ageing of the trees. Numerically there is a difference, but statistically there are significant differences (P-value꞊0.001) between the carbon stored in dead wood lying on the ground and the carbon stored in standing dead wood.
Coarse woody decomposition class
In the Mipoutou area, we observed an abundance of class II standing deadwood, probably due to climatic disturbances. We also observed an abundance of dead wood lying on the ground belonging to class I, which is probably due to local factors such as chablis, trees that fall due to natural death, or the action of violent winds that precede heavy rains, our work goes hand in hand with that of Ifo AS. et al., (2018). It may also be due to the fact that the Likouala department, more precisely the Ipendja FMU, is characterized by heavy rainfall throughout the year, with humidity approaching 95% and an average temperature of 25°C, according to data from the Impfondo meteorological station, which favours the decomposition of dead wood. It may also be due to the colonization of termites, fungi, saproxylic insects (which eat decomposing dead wood) and xylophagous beetles, which accelerate the decomposition of dead wood.
Our study concurs with that from Harmon ME. et al., (1986), which shows that xylophagous beetles had an important role to play in the decomposition process of dead wood. Compare with the results of Ndzaï SF. (2022), who obtained 7.55±2.15 t C ha-1 in the mature Celtis adolfi-friderici forest. This increase is certainly due to the presence of large diameters of dead wood in this class. The increase in carbon stock in this forest is certainly due to its remoteness from the village, where firewood collection is very rare. However, we found a higher carbon stock of 90.48 t C ha-1 in the dead wood lying on the ground. As for standing deadwood, our results show a storage rate of 7.28 t C ha-1. This difference is undoubtedly due to the windfall phenomenon, which raises the carbon content of dead wood lying on the ground.
Diameter class
The variability of carbon stock in dead wood depends mainly on diameter classes. In all the deadwoods studied, the greatest quantities of carbon are found in the larger-diameter sample classes. Our results clearly show that the diameter classes with the largest number of samples are those that store the most carbon. For standing deadwood, the lowest stock is obtained in classes X and XI with 0 t C ha-1, i.e. 2 samples. On the other hand, the highest stock was obtained in class XII with 29.06 t C ha-1, i.e. 9 samples. For dead wood lying on the ground, class XII had the highest number of samples, with 177.48 t C ha-1 or 22 samples.
Class I, on the other hand, has the lowest number of samples, with 1.78 t C ha-1 or 20 trees. This study shows that carbon stock depends on the number of samples. These results are not in line with those of Ndzaï SF. (2022), who obtained the highest carbon stock in forests with fewer samples than in those with a huge number of samples. In the Lophira alata flooded mature forest, the lowest stock was obtained in class I with 14.41 t C ha-1, i.e. 303 samples. By contrast, the highest stock was obtained in class VII (50.82 t C ha-1, or 13 samples). In the Celtis adolfi-friderici mature forest, class I had the highest number of trees (17.43 t C ha-1, or 353 samples), while class VII had 10 samples with a high stock (43.93 t C ha-1).
The Guibourtia demeusei flooded adult forest displays 13.5 t C ha-1 in class I for 251 samples of large woody debris, with the highest carbon stock in class VI (82.06 t C ha-1 with 28 trees). In the Macaranga monandra Secondary Forest, class I has the highest number of samples, 425, with a low carbon stock (8.05 t C ha-1). However, class VII with 5 samples stored more carbon (13.92 t C ha-1). In the Musanga cecropioïdes Secondary Forest, class II had a high carbon stock (31.13 t C ha-1 and 310 samples). This study shows that carbon stock does not depend on the number of trees, but on tree diameter and specific density (Henry M. et al., 2010). Large-diameter trees (Doetterl S. et al., 2015 and Fayolle A. et al., 2016) constitute significant carbon stocks (trunk, branches, large roots). Clark and Clark (2000) point out that trees with a DBH ≥ 79 cm contribute around 30% to a higher carbon stock.
Conclusion
A study to estimate the carbon stock of dead wood in the Ipendja Forest Management Unit (FMU), Likouala Department, enabled us to take stock of our knowledge of the dead wood compartment in terms of its type, decomposition class and diameter class, and the role it plays in the functioning of forest ecosystems in terms of carbon quantification. This study shows that dead wood lying on the ground has a higher carbon stock than standing dead wood. Furthermore, this study demonstrates that the deadwood compartment must be taken into account in quantifying the carbon stock of forest ecosystems. It should be noted that very little information is available about the carbon stock of coarse woody debris in the Republic of Congo.
Acknowledgement
The authors would like to thank STC (Société Thanry Congo) for supporting this work. We greatly thank LGETA (Laboratoire de Géomatique et d’Ecologie Tropicale Appliquée) for its valuable contribution regarding this study. National Higher School of Agronomy and Forestry from Université Marien Ngouabi also supported this study. Different anonymous referees have provided substantial contribution and the authors address to them their heartfelt thanks.
Conflict of Interest
The authors declare no conflicts of interest.
References
Ifo, A. S. (2010). Carbon input to the soil and stock in two forest types (gallery forest and secondary forest) of the Téké plateaus. Université-Marien-NGouabi, Brazzaville 194.
Google Scholar, Cross Ref, Indexed at
Ekoungoulou, R., Mikouendanandi, M. R. B. E., Liu, X. D. (2021). Carbon storage in an intact republic of Congo’s forest. Applied Ecology And Environmental Research 19:439-451.
Google Scholar, Cross Ref, Indexed at
Zoghaib, R. (2021). Carbon stock assessment in Lebanese forests. Example of the forests of Nahr Beirut (Doctoral dissertation, Normandy University; Saint-Joseph University (Beirut).
Moorhead, J., Nixon, T. (2016). Global 500 Greenhouse Gases Performance 2010-2015. Thomson Reuters.
Baker, T. R., Chao, K. J. (2011). Manual for coarse woody debris measurement in RAINFOR plots. Leeds UK.
Lewis, S. L., Sonké, B., Sunderland, T., Begne, S. K., Lopez-Gonzalez, G., Van Der Heijden, G. M., Zemagho, L. (2013). Above-ground biomass and structure of 260 African tropical forests. Philosophical Transactions of the Royal Society B: Biological Sciences 368:20120295.
Google Scholar, Cross Ref, Indexed at
Ifo, SA, Binsangou, S., Mbemba, M. (2018). Decomposition of large woody debris in the tropical rainforests of the Congo Basin. International Journal of Biological and Chemical Sciences 12:837-849.
Google Scholar, Cross Ref, Indexed at
Ekoungoulou, R., Nzala, D., Liu, X., & Niu, S. (2018). Ecological and structural analyses of trees in an evergreen lowland Congo basin forest. International Journal of Biology 10:31-43.
Google Scholar, Cross Ref, Indexed at
Pan, Y., Birdsey, R. A., Fang, J., Houghton, R., Kauppi, P. E., Kurz, W. A., Hayes, D. (2011). A large and persistent carbon sink in the world’s forests. Science 333:988-993.
Google Scholar, Cross Ref, Indexed at
Van der Werf, G. R., Morton, D. C., DeFries, R. S., Olivier, J. G., Kasibhatla, P. S., Jackson, R. B., Randerson, J. T. (2009). CO2 emissions from forest loss. Nature Geoscience 2:737-738.
Google Scholar, Cross Ref, Indexed at
Ekoungoulou, R. (2014). Carbon Stocks Evaluation in Tropical Forest, Congo. LAP LAMBERT Academic Publishing.
Ekoungoulou, R., Niu, S., Loumeto, J. J., Ifo, S. A., Bocko, Y. E., Mikieleko, F. E. K., Liu, X. (2015). Evaluating the carbon stock in above-and below-ground biomass in a moist central African forest. Applied Ecology and Environmental Sciences 3:51-59.
Google Scholar, Cross Ref, Indexed at
Pascal, JP (2003). Notions on the structure and dynamics of tropical rainforests. French Forestry Review 55:118-130.
Penman, J., Gytarsky, M., Hiraishi, T., Krug, T., Kruger, D., Pipatti, R. Wagner, F. (2003). Good practice guidance for land use, land-use change and forestry.
Bocko, Y. E., Ifo, S. A., Loumeto, J. J. (2017): Quantification of carbon stocks in three key carbon pools in Central Africa: Case of the Likouala Swamp Forest (Northern Congo). European Scientific Journal, 13(5): 438-456.
Ekoungoulou, R., Nzala, D., Liu, X., & Niu, S. (2018). Tree biomass estimation in central African forests using allometric models. Open Journal of Ecology 8:209-237.
Google Scholar, Cross Ref, Indexed at
Ndzaï, S.F. (2022): Characterization and estimation of carbon stocks in adult and secondary tropical forests in the Impfondo-Dongou zone, Likouala Department. Doctoral thesis. Forest Botany. ENSAF, Marien NGOUABI University, Brazzaville, Republic of Congo.
Baker, T. R., Honorio Coronado, E. N., Phillips, O. L., Martin, J., Van Der Heijden, G. M., Garcia, M., Silva Espejo, J. (2007). Low stocks of coarse woody debris in a southwest Amazonian forest. Oecologia 152:495-504.
Google Scholar, Cross Ref, Indexed at
MEF. (2021): Focus on the forest in the Republic of Congo. Ministry of Forest Economy, Brazzaville, Congo.
Abbe, T. B., Montgomery, D. R. (1996). Large woody debris jams, channel hydraulics and habitat formation in large rivers. Regulated Rivers: Research & Management 12:201-221.
Google Scholar, Cross Ref, Indexed at
Wohl, E. (2011). What should these rivers look like? Historical range of variability and human impacts in the Colorado Front Range, USA. Earth Surface Processes and Landforms 36:1378-1390.
Google Scholar, Cross Ref, Indexed at
Montgomery, D. R., Buffington, J. M., Smith, R. D., Schmidt, K. M., Pess, G. (1995). Pool spacing in forest channels. Water Resources Research 31:1097-1105.
Google Scholar, Cross Ref, Indexed at
Sear, D. A., Millington, C. E., Kitts, D. R., Jeffries, R. (2010). Logjam controls on channel: Floodplain interactions in wooded catchments and their role in the formation of multi-channel patterns. Geomorphology, 116:305-319..
Google Scholar, Cross Ref, Indexed at
STC. (2022): Annual report of the company Thanry Congo. Vic-wood group. Ipendja, Republic of Congo.
ANAC. (2022): Directorate of Meteorology, Brazzaville station (Maya-Maya), average temperature and precipitation for the period from 2016 to 2021. National Civil Aviation Agency. Brazzaville, Congo.
Pedlar, J. H., Pearce, J. L., Venier, L. A., McKenney, D. W. (2002). Coarse woody debris in relation to disturbance and forest type in boreal Canada. Forest Ecology and Management 158:189-194.
Google Scholar, Cross Ref, Indexed at
Clark, D.F., Kneeshaw, D.D., Burton, P.J., Antos, J.A. (1998). Coarse woody debris in sub-boreal spruce forests of west-central British Columbia. Canadian Journal of Forest Research 28:284-290.
Google Scholar, Cross Ref, Indexed at
Laiho, R., Prescott, C. E. (1999). The contribution of coarse woody debris to carbon, nitrogen and phosphorus cycles in three Rocky Mountain coniferous forests. Canadian Journal of Forest Research 29:1592-1603.
Google Scholar, Cross Ref, Indexed at
Warren, W., Olsen, P. F. (1964). A line intersect technique for assessing logging waste. Forest Science 10:267-276.
Google Scholar, Cross Ref, Indexed at
Mund, M. (2004). Carbon Pools of European Beech Forests (Fagus Sylvatica) Under Different Silvicultural Managementorest (Doctoral dissertation, Forschungszentrum Waldökosysteme).
Harmon, M. E., Franklin, J. F., Swanson, F. J., Sollins, P., Gregory, S. V., Lattin, J. D., Cummins, K. W. (1986). Ecology of coarse woody debris in temperate ecosystems. Advances in ecological research 15 :133-302.
Google Scholar, Cross Ref, Indexed at
Henry, M., Besnard, A., Asante, W. A., Eshun, J., Adu-Bredu, S., Valentini, R., Saint-André, L. (2010). Wood density, phytomass variations within and among trees, and allometric equations in a tropical rainforest of Africa. Forest Ecology and Management 260 1375-1388.
Google Scholar, Cross Ref, Indexed at
Doetterl, S., Kearsley, E., Bauters, M., Hufkens, K., Lisingo, J., Baert, G., Boeckx, P. (2015). Aboveground vs. belowground carbon stocks in African tropical lowland rainforest: Drivers and implications. PloS One 10:e0143209.
Google Scholar, Cross Ref, Indexed at
Fayolle, A., Panzou, G. J. L., Drouet, T., Swaine, M. D., Bauwens, S., Vleminckx, J., Doucet, J. L. (2016). Taller trees, denser stands and greater biomass in semi-deciduous than in evergreen lowland central African forests. Forest Ecology and Management 374:42-50.
Google Scholar, Cross Ref, Indexed at
Chave, J., Réjou-Méchain, M., Búrquez, A., Chidumayo, E., Colgan, M. S., Delitti, W. B., Vieilledent, G. (2014). Improved allometric models to estimate the aboveground biomass of tropical trees. Global change biology, 20:3177-3190.
Google Scholar, Cross Ref, Indexed at
CNIAF. (2015): Map of forest cover change in the Republic of Congo for the period 2000-2012. National Center for Forest and Wildlife Inventory and Management.
Ekoungoulou, R., Niu, S., Folega, F., Nzala, D., Liu, X. (2018). Carbon stocks of coarse woody debris in Central African tropical forests. Sustainability in Environment 3:142.
Google Scholar, Cross Ref, Indexed at
Author Info
Romeo Ekoungoulou1*, Bienfaite Jariya Bamissamou1, Saint Fedriche Ndzaï1, Fousseni Folega2, Chrisveil de Ben-Mack Mbouilou1, Jafrel Lo�ck Bikouta1 and Felix Koubouana12Department of Botany and Plant Ecology, Faculty of Sciences, University of Lomé, 01BP1515 Lomé, Togo
Citation: Ekoungoulou, R., Bamissamou, BJ., Ndzaï, SF., Folega, F., Mbouilou, CBM., Bikouta, JL., Koubouana, F. (2025). Carbon stock estimation for coarse woody debris in a northern Republic of Congo’s forest. Ukrainian Journal of Ecology. 15:1-11.
Received: 11-Mar-2025, Manuscript No. UJE-25-162297; , Pre QC No. P-162297; Editor assigned: 13-Mar-2025, Pre QC No. P-162297; Reviewed: 11-Apr-2025, QC No. Q-162297; Revised: 23-Apr-2025, Manuscript No. R-162297; Published: 01-May-2025, DOI: 10.15421/2025_612
Copyright: This work is licensed under a Creative Commons Attribution 40 License
