Research Article - (2020) Volume 10, Issue 2
Abstract
We determined the histochemical activity of phosphatases of sweat and sebaceous glands in the withers of three artiodactyls species — roe deer Capreolus capreolus Linnaeus, 1758, musk deer Moschus moschiferus Linnaeus, 1758, and Saiga tatarica Linnaeus, 1766. Equal values of acid phosphatase activity (60% level of relative activity), alkaline phosphatase (80% level of relative activity), and adenosine triphosphatase (100% level of relative activity) for both the sweat and sebaceous glands of roe deer founded. Only for alkaline phosphatase, a 60% level of relative activity founded in the sweat glands of musk deer, while the remaining parameters of phosphatase activity in both types of skin glands of musk deer showed zero values. Moderate levels of activity of acid phosphatase and alkaline phosphatase (60% level of relative activity), as well as an average level of adenosine triphosphatase activity (80% level of relative activity) are typical for both the sweat and sebaceous glands of saiga. Synchronicity in the parameters of histochemical activity in both the sweat and sebaceous glands revealed, which may be associated with physiological adaptation. Coordinate analysis based on digital analogues of the histoenzymatic activity of the skin glands made it possible to calculate the Euclidean distances between the studied species. In the sweat and sebaceous glands the following individual distances identified: (1) distances between roe deer and musk deer are 5.92 and 7.07; (2) distances between the roe deer and saiga are coincide and equal to 1.41 and 1.41; (3) distances between musk deer and saiga are 5.00 and 5.83. Phenetic analysis, which includes 18 parameters of histoenzymatic activity — three types of phosphatases in the sweat and sebaceous glands of three species of artiodactyls — proximity of the values of roe deer and saiga at an obvious distance of the parameters of musk deer revealed. The Euclidean distances for the three species of artiodactyls were: (1) distance between the roe deer and musk deer is 9.22; (2) distance between roe deer and saiga is 2.00; (3) distance between musk deer and saiga is 7.68.
Keywords
Histochemistry; Phosphatases; Analytical geometry; Cluster analysis; Artiodactyls; Ruminants; Pecora; Roe deer; Musk deer; Saiga
Introduction
Research of the histochemistry of mammalian cutaneous gland enzymes is an urgent biological task, which allows expanding the understanding of the physiology of the glandular apparatus of the skin (Sokolov et al., 1988), the main function of which is to produce a secret that has an important functional value and ensures homeostasis of the animal’s body (Sokolov, 1982; Quay, 1986; Sokolov & Chernova, 2001). That is why close attention should be paid to the study of enzyme systems involved in secretion (Saga, Morimoto, 1995; Saga, 2001, 2002; Cui & Schlessinger, 2015), while the determination of histoenzymatic activity will allow us to characterize the physiological parameters of the sweat and sebaceous glands (Dzhemukhadze, 2007), which belong to modular systems of the skin with different morphological structures (Chernova & Kiladze, 2019). The sweat glands located in the thickness of the skin have a tubular structure ending in a glomerulus, and the sebaceous glands adjacent to the hair are characterized by a bunch-like shape (Hashimoto et al., 1986; Bell, 1986; Sokolov & Petrishchev, 1997; Sokolov & Chernova, 2001). It is believed that the secret of sweat and sebaceous glands encodes both reactively changing conditions of the body and, on the contrary, characterizes more stable physiological parameters of an individual (Dzhemukhadze, 2007). It is obvious that the histochemistry and cytochemistry of enzymes requires an extension of the data processing methods used, previously presented in the descriptive-symbolic form (Montagna & Noback, 1947; Tuzuner & Bennett, 2018). Translation of signs indicating the gradation of enzyme activity into digital analogues allows using the methods of qualimetry, statistics and mathematics, which provides not only the possibility of identifying relationships that can be discussed from a biological point of view, but also gives the necessary rigor and evidence of the obtained histochemical results (Dzhemukhadze & Kiladze, 2008; Azgaldov & Kostin, 2011; Lobanov, 2013).
In the class Mammalia system, the order Artiodactyla occupies a significant place, many of which have a certain hunting, commercial and economic significance, while some species are under national and even international protection because of their vulnerable status (Price & Gittleman, 2007). We have studied the skin cover in the withers of such species as roe deer, musk deer, and saiga, while it is believed that the world population of musk deer (230 thousand individuals) and saiga (165 thousand individuals) tends to decrease, which allowed the International Union for Conservation of Nature and Natural Resources (IUCN Red List) to give quite alarming statuses for these species — Vulnerable A2d+3d+4d and Critically Endangered A2acd, respectively (Siberian Musk Deer…, 2012; Nyambayar et al., 2015; IUCN SSC…, 2018; Vaughan, 2019). The main products of hunting for musk deer are the musk deer jet, obtained from the preputial gland and used in the perfume industry (Sokolov et al., 1987; Sokolov & Chernova, 2001; Prikhod’ko, 2003; Yi et al., 2020), while the saiga have the main value in the horns used in traditional Chinese medicine (Vaughan, 2019; Doughty et al., 2019). Against this background, the global roe deer population is quite large
(15 million individuals), which allows for quite active hunting and commercial activities (Lovari et al., 2016), however, in this case, environmental management of the population of these artiodactyls is also required (Marcon et al., 2019), even with the current favorable status (Least Concern). Hunting for roe deer is carried out because of the meat fraction, skins that go to suede production, as well as trophy horns (Spiers, 1973; Weiner, 1973; Kuznetsov, 2005; Khludeev & Gordienko, 2008).
The purpose of this work is to research the histoenzymatic activity of the sweat and sebaceous glands of three representatives of the order Artiodactyla, belonging to the suborder Ruminantia and the infraorder Pecora, however, and the studied species are representatives of three different families, namely: (i) family Cervidae includes the roe deer Capreolus capreolus Linnaeus, 1758,
(ii) family Moschidae includes the musk deer Moschus moschiferus Linnaeus, 1758, and (iii) family Bovidae includes the saiga Saiga tatarica Linnaeus, 1766 (Pavlinov & Khlyap, 2012).
On the one hand, this circumstance in a certain way will show the species variability of histoenzymatic activity, and on the other hand, will allow to compare the obtained data with each other due to the taxonomic affinity of species using a qualimetric scale (Azgaldov et al., 2015; Rozhkov, 2018), a three-dimensional coordinate system (Aljohani, 2016), and also cluster analysismethods (Gower, 1967), which are based on the phenetic parameters of enzyme activity of the skin glands. Previously, these methods we tested on the example of rodents (Kiladze & Dzhemukhadze, 2019, 2020) which makes them universal for the
analysis of various groups of animals.
Materials and Methods
Following species of artiodactyls selected as the object of the research (harvest wildlife locations are indicated in brackets): (1)roe deer C. capreolus (Belarus), (2) musk deer M. moschiferus (Eastern Siberia, Russia), and (3) saiga S. tatarica (Kazakhstan), belonging to the same sex (males), age (adult mature individuals) and hunting season (November-December). For histochemical analysis, sweat and sebaceous glands of the skin from the withers researched. Three skin samples (1×1 cm) obtained from each individual in 10% neutral formalin solution for up to 24 hours in the cold fixed. The thickness of the frozen―floating‖ slides was 15 μm (Pears, 1960; Barca & Anderson, 1963). Histochemical reactions to acid phosphatase (ACP), alkaline phosphatase (ALP), and adenosine triphosphatase (ATPase) performed according to Gomori and Burstone (Gomori, 1952; Burstone, 1962). The microscopic examination of the histological slides carried out using a Carl Zeiss microscope (manufacturer VEB Carl Zeiss Jena, Germany).Digital data processing involves the creation of a qualimetric scale of enzyme activity. In this regard, the ranking of the activity ofskin gland enzymes carried out with a sign, digital and relative representation of the results, namely: – /0 points /0% — traces or absence of activity; − (+) /1 point / 20% — indistinct activity; + (−) /2 points /40% — low activity; + / points /60% — moderate activity; ++ /4 points /80% — average activity; +++ /5 points /100% — high activity (Kiladze & Dzhemukhadze, 2019, 2020).
The relative characteristic of enzyme activity is defined as the ratio of actual activity to maximum activity, with the result expressed as a percentage (Kiladze & Dzhemukhadze, 2013).
Digital analogues of the histochemical activity of the sweat and sebaceous glands of roe deer, musk deer and saiga presented in the form of coordinates in three-dimensional space, where the activity of ACP plotted on the x-axis, ALP plotted on the y-axis, ATPase plotted on the z-axis (Kiladze & Dzhemukhadze, 2019, 2020). Three-dimensional graphs created using the program ―CPM 3D Plotter‖, available at https: //technology.cpm.org/general/3dgraph/.
Euclidean distances for the histochemical parameters of sweat and sebaceous glands determined between roe deer (A) and musk deer (B), roe deer (A) and saiga (B), and musk deer (A) and saiga (B), while the distances (d) determined as follows way:

The automated calculation of distances (d) performed using the site: http: //www.mathsolution.ru/math-task/lp-dist-2points. For the phenetic constructions, the initial data were digital analogs of the activity of three types of phosphatases for two types of glands from three types of artiodactyls, that is, 18 histochemical parameters that were subjected to clustering by Ward’s method using the computer program ―STATISTICA 10‖ (StatSoft, USA).
Results
Given the environmental relevance of the research objects, as well as their significant commercial potential, great importance is given to the research of general biological patterns of artiodactyls, which can include features of the functioning of the skin glands, the physiological aspects of which are largely related to enzyme systems.
Histoenzymatic analysis of the sweat and sebaceous glands of the skin of the withers of roe deer, musk deer, and saiga suggests the identification of gradations of activity of the three types of phosphatases, shown both in sign form and in the form of digital analogues (Table 1), determined by the qualimetric scale.
| Type of phosphatase | Roe deer C. capreolus |
Musk deer M. moschiferus |
Saiga S. tatarica |
|||
|---|---|---|---|---|---|---|
| Sweat glands | Sebaceous glands | Sweat glands | Sebaceous glands | Sweat glands | Sebaceous glands | |
| Acid phosphatase, points | + / 3 | + / 3 | – / 0 | – / 0 | + / 3 | + / 3 |
| Alkaline phosphatase, points | ++ / 4 | ++ / 4 | + / 3 | – / 0 | + / 3 | + / 3 |
| Adenosine triphosphatase,points | +++ / 5 | +++ / 5 | – / 0 | – / 0 | ++ / 4 | ++ / 4 |
Table 1. Histoenzymatic activity of phosphatases of cutaneous glands in the withers of the studied artiodactyls.
Analyzing the above results, we can indicate that the same activity levels of ACP, ALP, and ATPase of roe deer for both sweat and
sebaceous glands founded. For ACP, a 60% level of the relative manifestation of the enzyme is characteristic, for ALP — 80%, for ATPase —100%. Musk deer is characterized by a minimal level of activity for all types of phosphatases in both sweat and sebaceous glands. The results obtained indicate only the presence of traces or the absence of an enzyme in the cells of the skin glands. A moderate level of activity was detected only for ALP in the sweat glands, which suggests a 60% relative character.
Moderate level of activity only for ALP in the sweat glands detected, which suggests a 60% relative nature of the histoenzymatic manifestations on the preparations. Moderate and average levels of ACP, ALP and ATPase are typical for the sweat and sebaceous glands of saiga, while the maximum level of enzyme expression in the preparations not detected. If ACP and ALP have a 60% relative value, then for ATPase it reaches 80% level.
Taking into account the key importance of phosphatases in the process of secretion, we modeled three-dimensional phosphatase spaces, where the values of the activity of ACP, ALP, and ATPase founded as coordinates, both in the sweat (Figure 1a) and in the sebaceous (Figure 1b) glands of roe deer, musk deer and saiga. Interpretation of the enzymes activity in the form of coordinates made it possible to calculate the Euclidean distance between the studied animal species, thereby showing the degree of proximity of the phosphatase activity for two types of glands of the skin (Table 2).
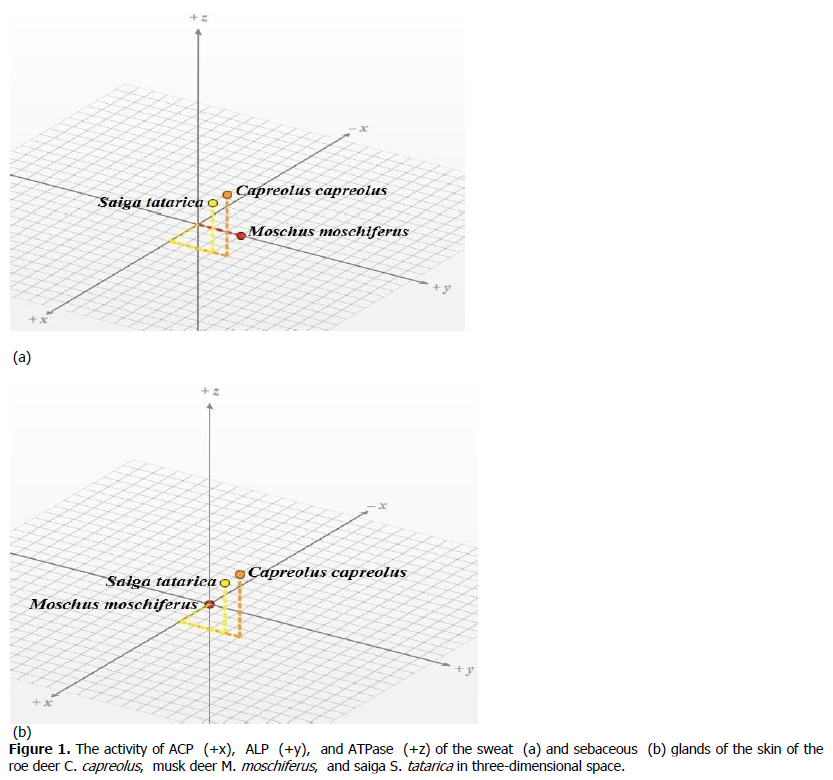
Figure 1. The activity of ACP (+x), ALP (+y), and ATPase (+z) of the sweat (a) and sebaceous (b) glands of the skin of the roe deer C. capreolus, musk deer M. moschiferus, and saiga S. tatarica in three-dimensional space.
| Pairwise comparison of species | Sweat glands | Sebaceous glands |
|---|---|---|
| Roe deer − Musk deer | d = 5.92 | d = 7.07 |
| Roe deer − Saiga | d = 1.41 | d = 1.41 |
| Musk deer − Saiga | d = 5.00 | d = 5.83 |
Table 2. Interspecific distances of histoenzymatic activity of phosphatases of sweat and sebaceous glands in the withers area of the studied artiodactyls.
Individual values of the activity distances of the three types of phosphatases indicate that both the sweat and sebaceous glands of the musk deer parameters are sharply different from the values of the activity of roe deer and saiga. The maximum distance is typical for sweat and sebaceous glands between the roe deer and musk deer, and the minimum distance that coincides for both types of glands is registered between roe deer and saiga. The distance values between musk deer and saiga are intermediate.
In support of the found regularities, a cluster analysis of the data performed, which can be considered as an assessment of the phenetic similarity of the phosphatase activity of three artiodactyl species (Figure 2).
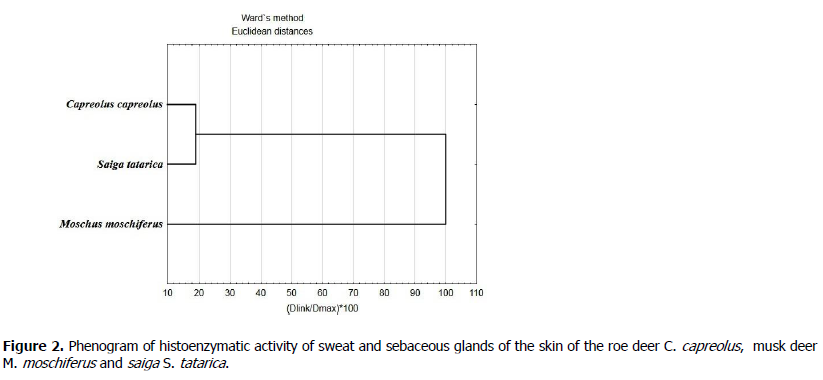
Figure 2. Phenogram of histoenzymatic activity of sweat and sebaceous glands of the skin of the roe deer C. capreolus, musk deer M. moschiferus and saiga S. tatarica.
The given phenogram, as well as the Euclidean distances shown in Table 3, indicate a significant distance between the parameters of the activity of musk deer phosphatases and the activity values of the enzymes of the roe deer and saiga skin glands, which are combined into a common cluster.
| Euclidean distances | C. capreolus | M. moschiferus | S. tatarica |
|---|---|---|---|
| C. capreolus | 0.00 | 9.22 | 2.00 |
| M. moschiferus | 9.22 | 0.00 | 7.68 |
| S. tatarica | 2.00 | 7.68 | 0.00 |
Table 3. Interspecific distances of histoenzymatic activity of phosphatases of skin glands in the withers area of the studied artiodactyls.
Discussion
Turning to the discussion of the results, it is important to indicate that the withers area of the three artiodactyl species can be considered as a model topographic area, characterized by a typical and stable level of functional activity of the skin glands (Sokolov & Petrishchev, 1997). Obviously, the revealed histochemical differences in the three representatives of artiodactyls are determined by species characteristics, as well as lifecycle and habitat (Burbaitė & Csányi, 2009; Wang et al., 2015; Slaght et al., 2019). In addition, attention is drawn to the synchronicity in the histoenzymatic characteristics for the two types of glands, which may indicate a similar intensity of secretion. Perhaps these levels of enzymatic activity in the sweat and sebaceous glands can be explained by the necessary physiological adaptation (Taylor, 1986; Gagnon et al., 2012). Qualimetric data processing allowed us to calculate the relative level of histoenzymatic activity from the maximum possible value, which made it possible to obtain information expressed as a percentage (Kiladze & Dzhemukhadze, 2013). The construction of phosphatase fields and drawing coordinates on them, which are digital analogues of the activity of ACP, ALP, and ATPase revealed a factorial effect of the type of skin glands on the enzymatic activity characteristic of the studied artiodactyl species.
Interpretation of histoenzymatic activity in the form of coordinates allowed using the Euclidean metric to calculate the distance between three species of animals for two types of glands. The results indicated that there are significant distances between the phosphatase activity of artiodactyls, since the values of musk deer are diametrically opposite to the level of activity of roe deer and saiga. These distances confirmed by cluster analysis, indicating similar trends in the phenetic parameters used. If compared the results with the taxonomic hierarchy of infraorder Pecora, we can fix the discrepancy, since the more closely related families are Cervidae, including roe deer, and Moschidae, including musk deer, while they somewhat distanced from Bovidae, which include the saiga (Pavlinov & Khlyap, 2012). It is obvious that the obtained results on the histochemistry of the cutaneous glands of three artiodactyl species should be considered only as an additional criterion for the species.
Conclusion
Thus, the final results can be formulated as the following theses: (1) species-specific parameters of the activity of ACP, ALP, and ATPase in the sweat and sebaceous glands of roe deer, musk deer, and saiga determined; (2) synchronicity of the histochemical activity of sweat and sebaceous glands in the withers area for the studied artiodactyl species revealed, which is possibly due to functional adaptation; (3) shows a significant distance in the histoenzymatic parameters of the activity of the skin glands of musk deer in comparison with roe deer and saiga, which was demonstrated both in coordinate space and using cluster analysis.
We believe that the data obtained using modern methods of qualimetric and biometric processing may be of interest for further comparative biological researches in the field of histochemistry and morphology of the skin glands of other mammalian groups.
References
Aljohani, S. (2016). Analytic Geometry of Three Dimensions. International Journal of Scientific and Engineering Research, 7 (4), 185−186.
Azgaldov, G., & Kostin, A. (2011). Applied qualimetry: its origins, errors and misconceptions. Benchmarking: An International Journal, 18 (3), 428–444. https: //doi.org/10.1108/14635771111137796
Azgaldov, G. G., Kostin, A. V., & Padilla Omiste, A. E. (2015). The ABC of Qualimetry: The toolkit for measuring immeasurable / interpreter E. Azgaldov. Ridero, Yekaterinburg. Barca, T., & Anderson, P. (1963). Histochemistry. Theory, Practice and Bibliography. Evanston, New York.
Bell, M. (1986). Sebaceous Glands. In: Bereiter-Hahn, J., Matoltsy, A. G., & Richards, K. S. (eds). Biology of the Integument. Springer, Berlin, Heidelberg, 318−338. https: //doi.org/10.1007/978-3-662-00989-5_18
Burbaitė, L., & Csányi, S. (2009). Roe deer population and harvest changes in Europe. Estonian Journal of Ecology, 58 (3), 169−180. DOI: 10.3176/eco.2009.3.02
Burstone, M. S. (1962). Enzyme Histochemistry and Its Application in the Study of Neoplasms. Academic Press, New York. Chernova, O. F., & Kiladze, A. B. (2019). Heterochrony as the basis for inter- and intraspecific diversity of skin in vertebrates. Biology Bulletin Reviews, 9 (2), 174–189. https: //doi.org/10.1134/S207908641902004X
Cui, C.‐Y., & Schlessinger, D. (2015). Eccrine sweat gland development and sweat secretion. Experimental Dermatology, 24 (9), 644−650. doi: 10.1111/exd.12773
Doughty, H., Verissimo, D., Tan, R. C. Q., Lee, J. S. H., Carrasco, L. R., Oliver, K., & Milner-Gulland, E. J. (2019). Saiga horn user characteristics, motivations, and purchasing behaviour in Singapore. PLoS ONE, 14 (9): e0222038. https://doi.org/10.1371/journal. pone.0222038
Dzhemukhadze, N. K. (2007). The dependence of interspecific differences in the histoenzymatic parameters of skin glands between Norway (Rattus norvegicus) and black (Rattus rattus) rats on their social behavior. Doklady Biological Sciences, 416 (1), 368–370. doi: 10.1134/S0012496607050122
Dzhemukhadze, N. K., & Kiladze, A. B. (2008). Comparison of the activity of some phosphatases in the midventral gland and nonspecific sebaceous glands of the neck in the Campbell hamster (Phodopus campbelli). Doklady Biological Sciences, 423 (1), 447–449. doi: 10.1134/S0012496608060239
Gagnon, D., Ganio, M. S., Lucas, R. A., Pearson J., Crandall C. G., Kenny G. P. (2012). Modified iodine-paper technique for the standardized determination of sweat gland activation. J Appl Physiol, 112, 1419–1425. doi: 10.1152/japplphysiol.01508.2011 Gomori, G. (1952). Microscopic Histochemistry: Principles and Practice. University of Chicago Press, Chicago.
Gower, J. C. (1967). A Comparison of Some Methods of Cluster Analysis. Biometrics, 23 (4), 623–637. DOI: 10.2307/2528417 Hashimoto, K., Hori, K., & Aso, M. (1986). Sweat Glands. In: Bereiter-Hahn, J., Matoltsy, A. G., & Richards, K. S. (eds). Biology of the Integument. Springer, Berlin, Heidelberg, 339−356. https: //doi.org/10.1007/978-3-662-00989-5_19
IUCN SSC Antelope Specialist Group (2018). Saiga tatarica. The IUCN Red List of Threatened Species 2018: e.T19832A50194357. http: //dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T19832A50194357.en. Downloaded on 07 January 2020.
Khludeev, K. D., & Gordienko, I. M. (2008). Tovarovedenie i ekspertiza kozhevennogo syriya [Commodity Research and Examination of Raw Hides and Skins]. Koloss, Moscow. (in Russian).
Kiladze, A. B., & Dzhemukhadze, N. K. (2013). Kvalimetriya v gistohimii fermentov (na primere kozhnyh zhelez mlekopitayushchih) [Qualimetry in the histochemistry of enzymes in mammalian skin glands]. Infra-Inzheneriya, Moscow. (in Russian).
Kiladze, A. B., & Dzhemukhadze, N. K. (2019). Evaluation of sexual dimorphism of histochemical activity of phosphatases of the plantar glands of Norway rats (Rattus norvegicus). Biosystems Diversity, 27 (1), 39–42. doi: 10.15421/011906
Kiladze, A. B., & Dzhemukhadze, N. K. (2020). Biokvalimetricheskij analiz aktivnosti fosfataz zhelez kozhnogo pokrova seryh i chernyh krys [Bioqualimetric study of the phosphatases activity of skin glands of Norway rats and black rats]. Institute of Computer Science, Moscow, Izhevsk, (In Russian).
Kuznetsov, B. A. (2005). Tovarovedenie vtorostepennyh vidov životnogo syriya [Commodity studies of secondary types of animal raw materials]. Aquarium-Print Ltd., Moscow. (in Russian). Lobanov, A. S. (2013). The basic concepts of qualimetry. Scientific and Technical Information Processing, 40, 72–82. https://doi.org/10.3103/S0147688213020044
Lovari, S., Herrero, J., Masseti, M., Ambarli, H., Lorenzini, R., & Giannatos, G. (2016). Capreolus capreolus. The IUCN Red List of Threatened Species 2016: e.T42395A22161386. http: //dx.doi.org/10.2305/IUCN.UK.2016- 1.RLTS.T42395A22161386.en. Downloaded on 07 January 2020.
Marcon, A., Battocchio, D., Apollonio, M., & Grignolio, S. (2019) Assessing precision and requirements of three methods to estimate roe deer density. PLoS ONE, 14 (10), e0222349. https: // doi.org/10.1371/journal.pone.0222349
Montagna, W., & Noback, C. R. (1947). Histochemical observations on the sebaceous glands of the rat. American Journal of Anatomy, 81, 39−61. doi: 10.1002/aja.1000810103
Nyambayar, B., Mix, H., & Tsytsulina, K. (2015). Moschus moschiferus. The IUCN Red List of Threatened Species 2015: e.T13897A61977573. http: //dx.doi.org/10.2305/IUCN.UK.20152.RLTS.T13897A61977573.en. Downloaded on 07 January 2020.
Pavlinov, I. Ya., & Khlyap, L. A. (2012). Order Artiodactyla. Pavlinov, I. Ya., & Lissovsky, A. A. (Eds.). The mammals of the Russia: A taxonomic and Geographic Reference (Archive of the Zoological Museum of MSU. Vol. 52). KMK Sci Press, Moscow, 429–473.
Pears, A. G. E. (1960). Histochemistry: Theoretical and Applied. 2nd ed. J. and A. Churchill Ltd., London.
Price, S. A., & Gittleman, J. L. (2007). Hunting to extinction: biology and regional economy influence extinction risk and the impact of hunting in artiodactyls. Proceedings of the Royal Society B: Biological Sciences, 274 (1620), 1845–1851. http://doi.org/10.1098/rspb.2007.0505
Prikhod’ko, V. I. (2003). Kabarga: proiskhozhdenie, sistematika, ekologiya, povedenie i kommunikatsiya [Musk Deer: Origin, Taxonomy, Ecology, Behavior, and Communication]. GEOS, Moscow. (in Russian).
Quay, W. B. (1986). Scent Glands. In: Bereiter-Hahn, J., Matoltsy, A. G., & Richards, K. S. (eds) Biology of the Integument. Springer, Berlin, Heidelberg, 357−373. https: //doi.org/10.1007/978-3-662-00989-5_20
Rozhkov, N. N. (2018). Kvalimetriya i upravlenie kachestvom. Matematicheskie metody i modeli [Qualimetry and quality management. Mathematical methods and models]. Yurayt Publishing House, Moscow. (in Russian).
Saga, K. (2001). Histochemical and immunohistochemical markers for human eccrine and apocrine sweat glands: an aid for histopathologic differentiation of sweat gland tumors. Journal of Investigative Dermatology Symposium Proceedings, 6 (1), 49–53. https: //doi.org/10.1046/j.0022-202x.2001.00005.x
Saga, K. (2002). Structure and function of human sweat glands studied with histochemistry and cytochemistry. Progress in Histochemistry and Cytochemistry, 37 (4), 323–386. DOI: 10.1016/s0079-6336 (02)80005-5
Saga, K., & Morimoto, Y. (1995). Ultrastructural localization of alkaline phosphatase activity in human eccrine and apocrine sweat glands. Journal of Histochemistry and Cytochemistry, 43 (9), 927–932. https: //doi.org/10.1177/43.9.7642965 SiberianMuskDeer−Moschusmoschiferus (2012). Available at: https: //web.archive.org/web/20121207003130/http://www.lhnet.org/siberian-musk-deer/ Accessed on 15 March 2020.
Slaght, J. C., Milakovsky, B., Maksimova, D. A., Seryodkin, I. V., Zaitsev, V. A., Panichev, A. M., & Miquelle, D. G. (2019). Anthropogenic influences on the distribution of a Vulnerable coniferous forest specialist: habitat selection by the Siberian musk deer Moschus moschiferus. Oryx, 53 (1), 174–180. doi: 10.1017/s0030605316001617 Sokolov, V. (1982). Mammal Skin. Berkeley: University of California Press.
Sokolov, V. E., & Chernova, O. F. (2001). Kozhnye zhelezy mlekopitayushchikh [Cutaneous Glands of Mammals]. GEOS, Moscow. (in Russian).
Sokolov, V. E., & Petrishchev, B. I. (1997). Kozhnyi pokrov domashnikh mlekopitayushchikh (kopytnykh) [The Skin of Domestic Ungulates]. Inst. Probl. Ekol. Evol., Ross. Akad. Nauk, Moscow. (in Russian). Sokolov, V. E., Kagan, M. Z., Vasilieva, V. S., Prihodko, V. I., & Zinkevich, E. P. (1987). Musk deer (Moschus moschiferus): Reinvestigation of main lipid components from preputial gland secretion. Journal of Chemical Ecology, 13, 71−83. https://doi.org/10.1007/BF01020352
Sokolov, V. E., Skurat, L. N., Stepanova, L. V., Sumina, E. B., & Shabadash, S. A. (1988). Rukovodstvo po izucheniyu kozhnogo pokrova mlekopitayushchih [Guide for Analysis of Skin of Mammals]. Nauka, Moscow. (in Russian).
Spiers, C. H. (1973). Deer skin leathers and their use for costume. Costume, 7 (1), 14–23. DOI: 10.1179/cos.1973.7.1.14
Taylor, N. A. S. (1986). Eccrine Sweat Glands. Sports Medicine, 3, 387–397. https: //doi.org/10.2165/00007256-198603060- 00001
Tuzuner, N. N., & Bennett, J. M. (2018). Classification of the Acute Leukemias: Cytochemical and Morphologic Considerations. In: Wiernik, P., Dutcher, J., & Gertz, M. (eds). Neoplastic Diseases of the Blood. Springer, Cham, 197−236 https://doi.org/10.1007/978-3-319-64263-5_14
Vaughan, A. (2019). Ban on saiga trade? New Scientist, 243 (3244), 12. doi: 10.1016/s0262-4079 (19)31559-3
Wang, W., Zhou, R., He, L., Liu, S., Zhou, J., Qi, L., Li, L., & Hu, D. (2015). The progress in nutrition research of musk deer: Implication for conservation. Applied Animal Behaviour Science, 172, 1–8. doi: 10.1016/j.applanim.2015.09.006
Weiner, J. (1973). Dressing percentage, gross body composition and caloric value of the roe-deer. Acta theriologica, 18 (11), 209−222.
Yi, L., Dalai, M., Su, R., Lin, W., Erdenedalai, M., Luvsantseren, B., Chimedtseren, C., Wang, Z., & Hasi, S. (2020). Whole-genome sequencing of wild Siberian musk deer (Moschus moschiferus) provides insights into its genetic features. BMC Genomics, 21, 108. https: //doi.org/10.1186/s12864-020-6495-2
Author Info
A.B. Kiladze* and N.K. DzhemukhadzeCitation: Kiladze, A.B., Dzhemukhadze, N.K. (2020). Comparative histochemical analysis of phosphatases activity of the skin glands in some artiodactyls. Ukrainian Journal of Ecology, 10 (2), 63-68.
Received: 17-Mar-2020 Published: 30-Jun-2020, DOI: 10.15421/2020_65
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
