Research - (2021) Volume 0, Issue 0
Degasation and dust control methods in major blasts in the open pit of inguletsky ore mining and processing complex (ingok). research and industrial tests results
Gerasimchuk Olexander1, Shchokin Vadym2*, Zamriy Sergii3 and Ezhov Vladislav4Abstract
The paper presents the results of experimental and industrial tests of the efficiency of reagents in the pre-humidification of units and their use in internal and external water stemming to reduce dust and degassing during mass explosions. In the course of the research from 2017 to 2021 in the conditions of PJSC "Ingulets Ore Mining and Processing Plant" reagents safe for humans and the environment were used, the maximum environmental efficiency of internal and external water stemming with the use of humate reagent TU U 20.5-43384697-001: 2020 was proved. The effective solution of humate reagent, which was used in the tests, was determined experimentally by conducting additional laboratory studies, the results of which are presented in the article. According to the results of industrial tests, the process of binding of fine dust particles, which are actively involved in the overall process of formation of the dust-gas cloud and the effect of neutralization of gases after the explosion is grounded. At a concentration of aqueous solution of humate reagent of 3%, the ecological efficiency in comparison with water was: dust suppression increased by 20.0%; neutralization of carbon monoxide-59.4%; neutralization of nitrogen oxides-55.1%.
Keywords
Open pit, Mass explosion, Humate reagent, Water stemming, Dust suppression, Degassing.
Introduction
Blasting in open pits is performed in separate blocks with single-row or multi-row arrangement of wells using different types of explosives (BP). The amount of explosives simultaneously detonated in one mass explosion reaches 300-1000 tons. During mass explosions, a significant amount of dust and harmful gases are produced, which in the form of a dust and gas cloud are carried out of the open pit space and scattered over long distances. At the same time, part of the harmful gases remains in the exploded rock mass, which in the process of its excavation is released into the atmosphere. According to the Research Institute of Occupational Safety and Ecology in the Mining and Metallurgical Industry (NDIBPG KNU) during mass explosions in open pits 0.027 to 0.170 kg of dust per 1 m3 of rock mass are produced (Shchokin, et al., 2018).
Known methods and measures to reduce dust and gas emissions into the atmosphere during mass explosions have not yet solved the above environmental problem, which is confirmed by the definition of degassing and dust suppression in mass explosions in open pits as one of the priorities for industrial enterprises in Kryvyi Rih. Scientific and Technical Ecological Council at the Department of Ecology and Natural Resources of Dnipropetrovsk Regional State Administration (item 1, Protocol No. 2 dated 09.06.2020), decisions of the Council of ecological planning at management of ecology of executive committee of Kryvyi Rih City Council (item 4.1 the Protocol from 27.10.2017, part II the Protocol from 30.06.2018) concerning implementation of measures of the City program of the decision of ecological problems of Kryvbas and improvement of a state of environment for 2016-2025 and others.
Objectives
The main methods of combating dust during mass explosions are currently based on the use of the method of pre-humidification of open pit blocks and the use of water stemming of various types. Types of water stemming are developed by NDIBPG KNU and include external, internal and combined. The "Guidance on the use of moistened stemming of charges in blasting operations in open pits, degassing of exploded rock mass and cleaning of the atmosphere from harmful products of the explosion" provides technological recommendations for the use of punches, organization of their use, and InGOK (2015) shows the effectiveness of measures for dust and gas suppression during mass explosions (Shchokin, et al., 2018).
When choosing a dust-binding reagent for effective dust suppression during mass explosions, it is also necessary to take into account its competitive cost and production conditions of the selected reagent in Ukraine.
The article presents the results of scientific work on determining the effective method and reagent for reducing dust and degassing during mass explosions. The problem was solved by conducting comprehensive industrial studies to determine the effectiveness of reducing dust formation and degassing when replacing water in the wells with reagents: anti-dust "Lexol-5", TU U 20.5-39086735-001: 2014, 3% aqueous solution; Reagent peat hydroxide (RTG) TU U 08.9-35-003: 2012 5% aqueous solution; mixture of RTG and VLR (carbon alkaline reagent) VLR-TU U 24.6.-24709453-001-2001 3% aqueous solution of X-ray and VLR; humate reagent, TU U 20.5-43384697-001: 2020 4% aqueous solution. As methods of use the most widespread are chosen: preliminary humidification of blocks; internal water stemming; external water stemming.
Methods
Working hypothesis
In order to theoretically substantiate the feasibility of using selected reagents for dust and gas suppression in mass explosions, a study of industrial adsorbents was performed, the main of which are activated carbon and its modifications. The use of carbon alkali reagent (HDR) and peat hydroxide reagent (RTG) in the working mixture during mass explosions in open pits showed its ability to sorb gases and dust. HRV is a product of brown coal processing, the main active components of HRV are sodium and potassium salts of humate acids and gelatinous substances, which are finely dispersed carbon-humate complexes. Common to humate acids of various origins is the presence of an aromatic nucleus and peripheral open chains, which consist of carboxyl, carbonyl groups, hydroxyls of alcohol and phenolic nature, the residue of nitrogen-containing amino acids. This structure of humate acids explains their adsorption properties. It is established that the process of adsorption of carbon monoxide (ll) VLR is an exothermic process and has a significant value of ΔH. It is equal to-179 kJ/mol, which allows us to conclude that there is chemisorption (not only physical but also chemical sorption). The ability to sorb can be explained by the structure of the CO molecule, which is extremely stable, but which has an atom of an element that can participate in the formation of a covalent bond by the donor-acceptor mechanism. An electron donor is an oxygen atom. Sorption of nitrogen oxides and ammonia is possible by a similar mechanism. In the case of ammonia, the donor is nitrogen (Tyschuk, et al., 2000), which allows us to conclude about the presence of chemisorption (not only physical but also chemical sorption). The ability to sorb can be explained by the structure of the CO molecule, which is extremely stable, but which has an atom of an element that can participate in the formation of a covalent bond by the donor-acceptor mechanism. An electron donor is an oxygen atom. Sorption of nitrogen oxides and ammonia is possible by a similar mechanism.
Effective neutralizers of carbon monoxide are potassium permanganate KMnO4 and hydrogen peroxide H2O2, which oxidize carbon monoxide CO to CO2 dioxide. NDIBPG staff proved that activated carbon undergoes physical sorption of gases, and its ability to absorb various gases, regardless of their chemical nature, has been experimentally confirmed.
Given these circumstances, to neutralize carbon monoxide, in the open pit of PJSC "InGOK" coal alkali reagent (LPR), which is a product of brown coal processing was selected as one of the test mixtures. The main active components of HRV are sodium salts of humate acids and gelatinous substances, which are finely dispersed carbon-humate complexes, and can be sorbents of CO molecules (Tishchuk, 2017).
One of the neutralizers of harmful gases is additionally selected peat hydroxide reagent, which is produced by treating peat with an aqueous solution of NaOH and belongs to the analogues of the carbon alkali reagent. The material is a dry fine powder containing more than 30% of sodium salts of humate acids (Yevdokimenko, et al., 2016). NDIBPG KNU conducted laboratory studies to determine the effectiveness of neutralization of harmful gases with an aqueous solution of peat hydroxide reagent in comparison with an aqueous solution of sodium humate (carbonaceous reagent). Laboratory tests were performed on a special laboratory stand according to the method developed by NDIBPG KNU. The absorption efficiency of carbon monoxide and sulphur (II) oxide by these solutions was determined by bubbling. To do this, gas, containing a fixed amount of harmful gases passed through the test solution and the efficiency of its purification was determined by the difference in gas concentrations, before passing through the solution and after that. Processing of measurement results was carried out by the method of mathematical statistics, according to GOST 8.207-76 "State system for ensuring the unity of measurements. Direct measurements with multiple observations. Methods of processing the results of observations. The main provisions." The concentration of carbon monoxide was determined using a MiniWarn gas analyser. For comparison, studies of 3% and 6% aqueous solutions of peat hydroxide reagent, 3% aqueous solution of carbon alkali reagent and 3% solution of hydrogen peroxide were performed (Yevdokimenko, et al., 2016).
Studies of the ability of humate reagent to sorb gases were carried out on a special stand. A gas containing carbon monoxide and sulphur dioxide was pumped through the test solution using an electric aspirator. The rate of gas passage through the reagent was equal to 0.2 dm3/min, the volume of the test gas made 2 dm3, the pumping time of was 10 minutes for one experiment. After passing a portion of gas through the humate reagent, the residual concentration of carbon monoxide (CO) and other components of the gas mixture was measured, namely: combustible gases and vapours (CnHm); nitrogen dioxide (NO2); sulphur dioxide (SO2); hydrogen sulphide (H2S). The difference in gas concentrations before (CO=830) and after passing through the humate reagent was determined by the amount of gas that was adsorbed by the reagent (Table 1).
| The concentration of humate reagent in aqueous solution, wt.%. | CO concentration before bubbling mg/m3 | The maximum concentration of CO after interaction with an aqueous solution of humate reagent, mg/m3 | The minimum concentration of CO after interaction with an aqueous solution of humate reagent, mg/m3 | Other components of the gas mixture | |||
|---|---|---|---|---|---|---|---|
| CnHm %about |
NO2 mg/m3 |
SO2 mg/m3 |
H2S mg/m3 |
||||
| 2 | 830 | 210 | 170 | 0.00 | 0 | 0 | 0 |
| 3 | 830 | 170 | 161 | 0.00 | 0 | 0 | 0 |
| 7 | 830 | 360 | 328 | 0.00 | 0 | 0 | 0 |
| 10 | 830 | 460 | 370 | 0.00 | 0 | 0 | 0 |
| 30 | 830 | 527 | 378 | 0.00 | 0 | 0 | 0 |
Table 1. The results of laboratory studies of the efficiency of gas absorption by the humate reagent.
Demonstration experiment on the adsorption of gases by a humate reagent from an internal combustion engine is given by reference, or QR-code (Fig. 1): http://nigri.dp.ua/gallery/video/V_R.mp4.

Fig 1. Video of a demonstration experiment.
According to the results of comparative laboratory studies, it was found that the humate reagent has the properties of absorbing harmful gases with components of carbon monoxide and sulphur dioxide. The minimum efficiency of neutralization of harmful gases with an aqueous solution of humate reagent: 2% solution: carbon monoxide 74.6-79.5%; 3% solution: carbon monoxide 79.5-80.6%; 7% solution: carbon monoxide 56.6-60.5%; 10% solution: carbon monoxide 44.6-55.4%; 30% solution: carbon monoxide 36.5-54.5%.
Results
An experimental explosions using anti-dust reagent surfactant "Lexol-5" was performed in the open pit belonging to the PJSC "InGOK" in September 2017 by NDIBPG KNU. The explosions were performed on the level-360 m. A typical diagram of one of the investigated open pit blocks, wet areas on the blocks and the place of installation of measuring equipment are shown in Fig. 2.
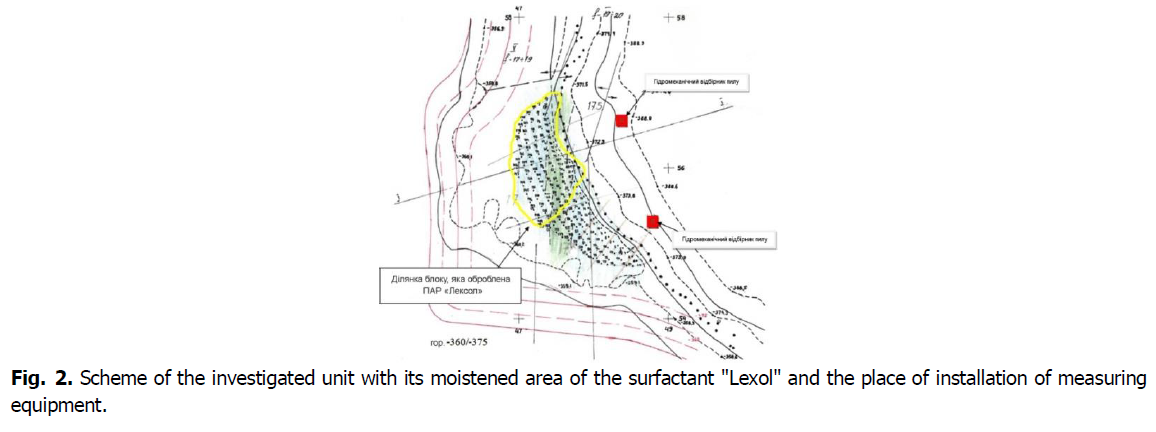
Fig 2. Scheme of the investigated unit with its moistened area of the surfactant "Lexol" and the place of installation of measuring equipment.
On the surface of the experimental section of the unit, an aqueous solution of surfactant "Lexol-5" was applied with a flow rate of 2.0 to 2.5 litres per 1 m2 of the investigated surface by spraying it with a watering machine with a hydromonitor. No dust suppressants were used in the second section.
The research confirmed the process of binding of the surfactant "Lexol" fine dust particles left on the surface of the blasting unit after drilling on its surface, and which are actively involved in the overall process of dust and gas cloud formation. At a concentration of an aqueous solution of anti-dust reagent "Lexol-5" 5%, the average efficiency of dust suppression was 21%.
Industrial studies have also shown the possibility of pre-moistening the open pit unit up to two days before the explosion. Evaporation of the applied reagent on the humidified block in the warm period of the year does not occur owing to formation of a protective film by reagent "Lexol-5".
Industrial tests during mass explosions in the open pit of PJSC "INGZK" were performed by NDIBPG KNU in June 2021 in order to determine the effectiveness of the method of dust and gas suppression by the method of external and internal water stemming, wet stemming with the use of humate reagent.
The studied block No.146 (Fig. 3) was located on the level-225 m, which was represented by magnetite quartzites.
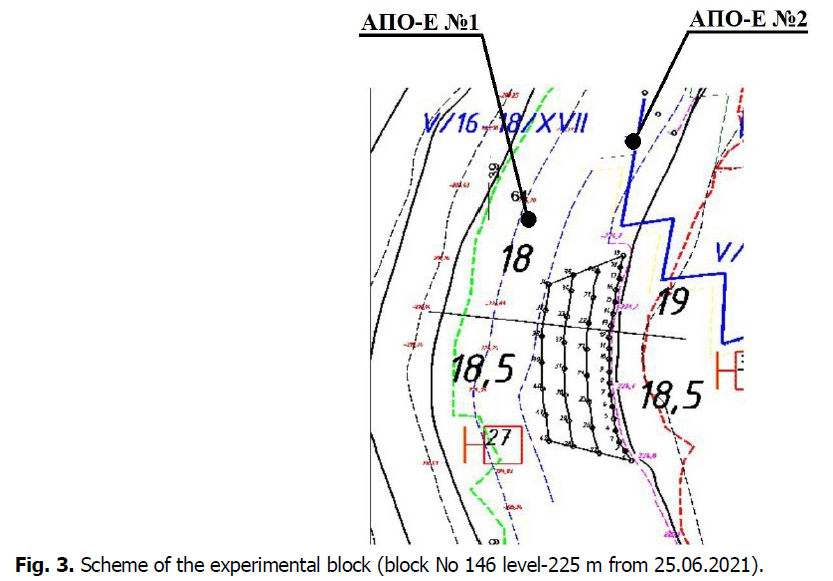
Fig 3. Scheme of the experimental block (block No 146 level-225 m from 25.06.2021).
Dust collectors are installed on each of the sections of the unit, which are located at a distance of 30-50 m from the last row of wells in the direction of the predominant movement of atmospheric air for 1.5-2 hours before the mass explosion. To ensure selective sampling from each section of the unit (sampling before the start of intensive mixing of dust and gas clouds from these sections of the unit), dust and gas collectors were placed at each section of the unit at the maximum possible distance. The location of dust and gas collectors is shown in Fig. 4.
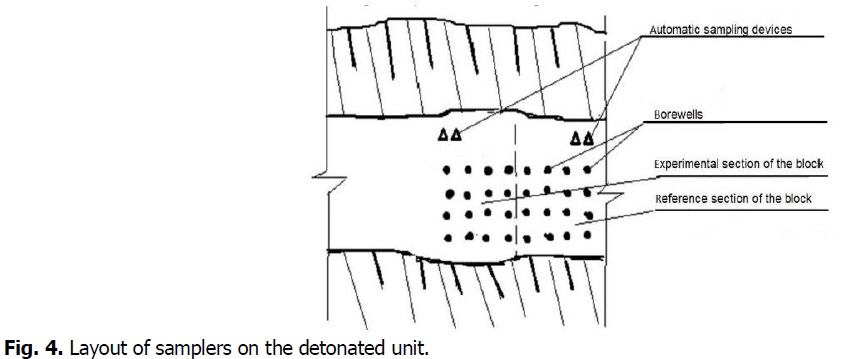
Fig 4. Layout of samplers on the detonated unit.
The analysis of dust and gas samples was carried out by express and laboratory methods in the analytical and testing laboratory of NDIBPG KNU, which meets the requirements of DSTU ISO 10012: 2005 "Measurement control systems. Requirements for measuring processes and measuring equipment" (certificate №08-0017/2018 dated 17.05.2018).
The effectiveness of the reagents when using an external water stemming is determined by the formula:
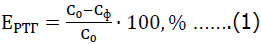
Where EPTT is the efficiency of dust and gas suppression when using the reagent, %; Co is the average concentration of harmful substances in the air without the use of reagent, mg/m3; Сф is-the average concentration of harmful substances in the air when using the reagent, mg/m3.
The amount of exploded rock mass on the blast block was 25,000 m3, the amount of explosives "Ukraine"-19490 kg, the number of wells-35 units. As methods of dust and gas suppression, the method of pre-wetting the surface of the unit was used and internal and external hydraulic stemming were used. Instead of water of technical quality, an aqueous solution of humate reagent with a concentration of 3% was used, which was prepared from a liquid 30% concentrate of humate reagent. The total volume of 3% aqueous solution was about 30 m3.
Dust extractors were placed at a distance of about 30-40 m from the last wells of the blasting unit. The scheme of the investigated unit and the location of dust and gas sampling devices are shown in Fig. 3.
Dust extractors are represented by automatic dust collectors of electric type APO-E (2 units). APO-E devices allow to carry out sampling on 1 filter like AFA and sampling of air in tight containers, with a capacity of 4,4 l. Further processing of selected air samples and weighing of filters was carried out in the laboratory of NDIBPG KNU.
The results of instrumental measurements of pollutant emissions during a mass explosion in the open pit of PJSC "INGZK", which was conducted on June 25, 2021 at block №146, level-255 m are shown in Table 2 and 3.
| Date of selection | Gas type | Concentration mg/m3 | The arithmetic mean concentration, mg/m3 |
Volume of dust and gas cloud, m3 | Specific emissions, kg/kg BP |
Total specific emissions, kg/kg BP |
|---|---|---|---|---|---|---|
| 25.06.2021 р. level-225 m bl. No.146 |
Calculation of gases in a dust and gas cloud | 0.00048 | ||||
| СО | 7.0 | 6.5 | 816655 | 0.00027 | ||
| СО | 6.0 | |||||
| Calculation of gases in the rock mass | ||||||
| СО | 567 | 550 | - | 0.00021 | ||
| СО | 533 | |||||
| Calculation of gases in a dust and gas cloud | 0.00014 | |||||
| NO2 | 3.0 | 2.5 | 816655 | 0.000105 | ||
| NO2 | 2.0 | |||||
| Calculation of gases in the rock mass | ||||||
| NO2 | 107.0 | 102 | - | 0.000039 | ||
| NO2 | 97.0 | |||||
Table 2. The results of calculations of gas emissions after a mass explosion in the open pit of PJSC "INGOK".
| Date, level, block | Data for calculating the concentration of dust on the detonated unit | The arithmetic mean concentration, mg/m3 |
Specific consumption of BP, kg/m3 | BP weight, kg | Volume of dust and gas cloud, m3 | Specific dust release | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Air flow according to the rotamete, l/min |
Air volume l | Sample on the filter mg |
Concentration, mg/m3 |
|||||||
| kg/m3 of rocks, which are blown up |
kg/kg of explosive | |||||||||
| 25.06.21 р. level.-225 m bl. No.146 |
5.0 | 5.45 | 1773,98 | 1782,12 | 0.78 | 19490 | 816655 | 0.0585 | 0.0747 | |
| 5.0 | 5.50 | 1790,25 | ||||||||
Table 3. The results of calculations of dust emissions after the explosion in the open pit of PJSC "INGOK".
According to the results of the measurements, the specific dust release was 0.0747 kg/kg/BP, gas evolution of: carbon monoxide 0.00048 kg/kg/BP, nitric oxide 0.00014 kg/kg/BP.
When calculating the effectiveness of dust and gas suppression measures used data from a mass explosion on 28.05.2021, approx. No.117, level-120 m. Specific indicators were: 0.159 kg/kg/BP, gas evolution of: carbon monoxide 0.00124 kg/kg/BP, nitric oxide 0.00031 kg/kg/BP.
Thus, the efficiency of using an aqueous solution of humate reagent, with a concentration of 3% in the internal and external water stemming was: dust suppression-53%; neutralization of carbon monoxide-61.3%; neutralization of nitrogen oxides-54.8%.
1) The volume of air reduced to normal conditions (temperature 273 K, pressure 101.3 kPa).
According to the results of industrial experiments and taking into account the multi-criteria approach to determine the technical and economic feasibility of environmental efficiency (Sobczyk, et al., 2017; Radwanek-Bąk, et al., 2020; Sobczyk, et al., 2021) promising measures to reduce emissions of pollutants into the atmosphere were developed. It has been experimentally proven that effective measures are: 1) pre-moistening of blasting blocks with an aqueous solution of 3% humate reagent, which reduces dust and neutralizes harmful gases after mass explosions during the year with positive temperatures. The efficiency of reducing dust when using this method is about 30%, the efficiency of neutralization of harmful gases (nitrogen oxides, carbon monoxide) is about 70%; 2) pre-humidification of the unit, which is blown up by 2-3% aqueous solution of humate reagent with an average consumption per 1 m2 of the surface of the unit 4-5l; 3) the use of aqueous solutions of humate reagent in internal and external water stemming. Industrial studies have shown the effectiveness of reducing dust in this measure to 38%, neutralization of harmful gases (nitrogen oxides, carbon monoxide) about 70%; 4) moistening the material of the inner face with a humate reagent. The calculation of the specific consumption of the reagent for humidification of the internal face is carried out under the condition of humidification to the optimum humidity and in accordance with the methodology of NDIBPG KNU is defined as: neutralization of harmful gases (nitrogen oxides, carbon monoxide) about 70%; 4) moistening the material of the inner face with a humate reagent. The calculation of the specific consumption of the reagent for moistening the internal face is carried out under the condition of moistening to the optimum humidity and in accordance with the method of NDIBPG KNU is defined as:
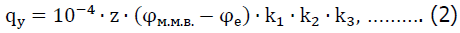
where-qy specific water consumption, m3/t; z is the content of the fraction with a size of 0-10 mm in 1 m3 of ore, rock, %; qy is the maximum molecular moisture content of ores or rocks of the fraction size 0-10 mm, %, φe is the natural humidity of rocks, %; k1 is the coefficient that takes into account evaporation from the surface of the rock mass, k1=1 for well conditions; k2 is the coefficient of non-uniformity of humidification, k2=1,2 1,2; k3 is the coefficient that takes into account the wetting of large pieces, k3=1 for well conditions.
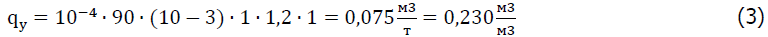
Under the condition of stemming in the well 1.4 m3, the flow of reagent per well will be 300-320 liters.
Dust reduction after mass explosions in the period with negative temperatures to-8°C is possible when using the method of pre-humidification of detonated units using aqueous solutions of surfactants "Lexol-5". The efficiency of reducing dust when using the proposed measure is approximately 20% (Table 4).
| S. No | Date of the study | Reagent used, TU | The physical state of the reagent supplied | Method of use | Dust suppression efficiency,% | Efficiency of neutralization of harmful gases, ,, % |
Estimated cost of reagent, UAH/t, UAH/m3 |
Supporting document |
|---|---|---|---|---|---|---|---|---|
| 1 | 08.09.2017 (block №146, level -360 m) |
Reagent antidust "Lexol-5", TU U 20.5-39086735-001: 2014, 3% aqueous solution |
20% concentrate in metal barrels | Pre-humidification | 21.2 | - | 12 500 UAH/t | Protocols of industrial research dated 08.09.2017 and 22.09.2017 |
| 22.09.2017 (block №166, level -360 m) |
||||||||
| 2 | 19.07.2019 (block №149 level-270 m) |
Peat hydroxide reagent (RTG) TU U 08.9-35-003: 2012 5% aqueous solution |
solid, in big bags |
Pre-humidification | 31 | 3700 UAH/t | Protocols of industrial research dated 07/19/2019 and 11/01/2019 |
|
| 11/01/2019 (block № 224, level -60 m) |
||||||||
| 3 | 18.09.2020 р. (block 90190, level-90/-105 m) |
A mixture of X-ray and HRV (carbon alkali reagent) VLR - TU U 24.6.-24709453-001-2001 3% aqueous solution of X-ray and HRV |
solid, in big bags |
Pre-humidification | 23 | VLR - 11900 UAH/t RTG - 3700 UAH/t mixture - 7800 UAH/t |
Protocols of industrial research dated 18.09.2020 and 27.11.2020 р. |
|
| 18.09.2020 р. (block №196, level-60/-75 m) |
||||||||
| 27.11.2020 р. (block №256, level -15 m) |
||||||||
| 4 | 28.05.2021 р. (block №117, level -120 m) |
The reagent humate, TU U 20.5-43384697-001: 2020 4% aqueous solution |
liquid 30% concentrate in tank trucks or Eurocubes | Internal and external hydraulic stemming, pre-humidification | 53 | 5500 UAH/m3 | Protocol of industrial research dated 28.05.2021 |
Table 4. Ecological efficiency of methods and reagents for dust suppression and neutralization of harmful gases during mass explosions in the open pit of PJSC "INGOK" within the period 2017-2021.
Conclusion
The use of pre-humidification of the blocks with an aqueous mixture of 3% solution of peat hydroxide reagent and 3% solution of carbon alkali reagent provided a reduction of dust by 23%, with degassing of harmful gases: carbon monoxide by 47%, nitrogen oxides by 54.0%.
The use of surfactant "Lexol-5" with pre-moistening of the unit with 5% aqueous solution provides an average efficiency of dust suppression of 21.2%, the effect of degassing is absent.
The efficiency of the use of humate reagent in the external water stemming in comparison with the use of technical water was: dust suppression increased by 33.0%; neutralization of carbon monoxide-61.3%; neutralization of nitrogen oxides-54.8% (no degassing effect in water).
To ensure the maximum environmental effect during mass explosions, it is necessary to use polyethylene containers with a diameter of about 0.3-1 m and more when forming an external water stemming of downhole charges of explosives. The external hammer in polyethylene sleeves is located on rows of wells. The length of the sleeves is determined by the geometric parameters of the surface of the charged unit and the contour of the wells. The technological process of performing an external hydraulic stemming with the use of a humate reagent does not differ from the standard implementation of a hydraulic stemming with the use of water.
In order to reduce dust emissions during mass explosions by more than 53%, reduce the impact of shock air waves and improve the quality of crushing rocks along the entire height of the ledge, it is advisable to form compacted to 2450 kg/m3 stemming of the inactive part of well charges BR 30% with humate concentrate and crushed rock with fraction 5-20mm.
References
Shchokin, V.P., Ezhov, V.V., Nalyvayko, V.G. (2018). Application of Lexol (R) surfactant aqueous solution to bind the dust on open pits roads and reduce the dust emission during large-scale blasts. Ukrainian Journal of Ecology, 8:755-761.
Typical measures to reduce emissions of pollutants into the atmosphere when using mass explosions in the open pit of PJSC "InGOK". (2015). Kryvyi Rih, p:3.
Tyschuk, V.Y., Ermak, L.D., Chasova, E.V. (2000). Kinetics of the process of gas-absorbing action of coal alkali reagent/Labor and environmental protection at the enterprises of the mining and metallurgical complex. Collection of Forged Works, Kryvyi Rih, pp:99-112.
Tishchuk, V.Yu. (2017). Influence of coke-chemical processes on air pollution of working zones and development of means of its clearing and improvement of working conditions/collection of scientific works of the National Mining University. The Dnieper, 51:226-233.
Yevdokimenko, M.F., Frantsev, E.V., Bondar, M.V., Kurinova, M.K. (2016). Investigation of carbon monoxide and sulfur dioxide uptake by peat hydroxide reagent during blasting operations in open pits. Proceedings of the International Scientific and Technical Conference "Development of Industry and Society". Krivyi Rih, 1:189.
Sobczyk, E.J., Kicki, J., Sobczyk, W., Szuwarzyński, M. (2017). Support of mining investment choice decisions with the use of multi-criteria method. Resources Policy, 51:94-99.
Radwanek-Bąk, B., Sobczyk, W., Sobczyk, E.J. (2020). Support for multiple criteria decisions for mineral deposits valorization and protection. Resources Policy, 68:1–11.
Sobczyk, W., Sobczyk, E.J. (2021). Varying the energy mix in the EU-28 and in Poland as a step towards sustainable development. Energies, 14:1502.
Author Info
Gerasimchuk Olexander1, Shchokin Vadym2*, Zamriy Sergii3 and Ezhov Vladislav42Research Mining Institute of the Krivoy Rog National University ul. Petra Doroshenko 11, 17. Krivoy Rog, Ukraine
3Industrial Safety and Environmental Protection Director of PJSC PJSC, Ingulets Ore Mining and Processing Plant ul. Rudna, 47 Krivoy Rog, Ukraine
4Research Institute of Labor Safety and Ecology in the Mining and Metallurgical Industry of the Krivoy Rog National University Pershotravneva Str.12, K, Ukraine
Citation: Olexander, G., Vadym, S., Sergii, Z., Vladislav, E. (2021). Degasation and dust control methods in major blasts in the open pit of inguletsky ore mining and processing complex (ingok). research and industrial tests results. Ukrainian Journal of Ecology 11 (8), 99-105.
Received: 20-Sep-2021 Accepted: 15-Oct-2021 Published: 25-Oct-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
