Research Article - (2021) Volume 11, Issue 2
Effect of maize residues and sawdust substrates on the growth and yield of oyster mushroom Pleurotus sapidus
A. K. Nasir1, T. W. Urwa1, A. Waqar1*, A. Ayesha2, A. Awais3, A. Asma1, K. Babar1 and M. Shahzad2Abstract
The cultivation of oyster mushrooms (Pleurotus sapidus) is considered a good environmentally friendly approach for the bio-conservation of agricultural residues into food. Mushroom is a good source of vitamins, amino acids, proteins and also contain less amount of fats cholesterol. P. sapidus is a heterotrophic organism and requires a nutritious substrate for growth. In this study, we evaluate the efficiency of maize residues (stalks, cobs, leaves) along with kikar tree (Vachellia nilotica) sawdust as a substrate on the growth, yield and biological efficiency of P. sapidus. Five treatments were prepared in different proportions, and data was recorded after spawn inoculation to the harvesting of mushrooms using various parameters like; spawn running, pinhead's formation, the number of pinhead's, development of fruiting bodies, yield, and biological efficiency. This study revealed that Treatment-T1 (sawdust 100%) significantly influenced most of the growth parameters compared with other treatments. Similarly, Treatment-T1 (sawdust 100%) produced maximum yield (444 g) and have maximum biological efficiency (88.8%), while Treatment-T5 (maize residues 100%) produced minimum yield (263 g) and have minimum biological efficiency (52.6%). We concluded that kikar tree sawdust is considered a potential substrate for the commercial cultivation of oyster mushrooms (P. spaidus).Keywords
Pleurotus sapidus, oyster mushroom, substrate, yield, biological efficiency
Introduction
Mushrooms are macro-fungi, have a heterotrophic mode of nutrition, spore-producing, and belongs to kingdom fungi (Kumar et al., 2015). Mushrooms are usually grown above ground on the soil or artificial medium used as substrate (Thakur, 2020). Oyster mushroom (Pleurotus sapidus) is an edible mushroom and is famous for its taste, flavor, and nutritional values. It is estimated that there are over 1.5 million fungal species are reported worldwide; among them, only 16,000 species are known as mushrooms, 700 species are known as medicinal mushrooms, and only 35 species are commercially cultivated for human consumption (Varghese and Amritkumar, 2020).
Mushrooms are gradually becoming an imperative constituent of the human's diet worldwide because of their medicinal properties and nutritional values (Khan et al., 2021). Mushroom is a good source of food to overcome the malnutrition problem in developing countries (Solovyev et al., 2018). Mushrooms have low starch contents and fats and are suitable for patients with diabetes and heart diseases (Devi et al., 2015). Oyster mushroom (P. sapidus) is a rich source of proteins contents (20-35%), less in fat contents (0.6-3%), moisture contents (70-90%), and fiber contents are ranges between (7.5-16.5%) (Jamil et al., 2019).
Oyster mushroom (Pleurotus spp.) is ranked third after button and shiitake mushroom globally and widely cultivated in Asia and Europe (Bhattacharjya et al., 2014). The average production of oyster mushrooms is about 40 million tons, and China is the largest producer and consumer of oyster mushrooms having a share (~90%) worldwide (Thakur, 2020). The average production of oyster mushrooms in Pakistan is about 200 tons, and 80% of total production is export to the USA and Europe (Jamil et al., 2019).
Cultivation of mushrooms is an excellent sustainable agriculture approach and is a food source of extra income for small farmers (Shirur and Shivalingegowda, 2015). Oyster mushrooms (P. sapidus) have simple, low-cost production technology and high biological efficiency. It is widely cultivated having the environmental condition; relative humidity 70-80%, temperate 20-30°C, and substrate pH 6-8 (Alananbeh et al., 2014). Preparation and selection of the substrate are the most critical factors that affect oyster mushrooms' growth and production. So, it is necessary to prepare a substrate-free from contamination (microorganisms) and rich in lignocellulosic and cellulosic substances (Sharma et al., 2017).
In Pakistan, in the regions of Khyber Pakhtunkhwa, Kashmir, and Punjab oyster mushroom is grown on different kinds of substrates like; cotton waste, wheat straw, paddy straw, and sawdust were used as a substrate for the commercial production of oyster mushrooms (Khan et al., 2017). Many studies have proven that cotton waste, wheat straw, and sawdust, along with cornflour and wheat bran, are considered suitable substrates for cultivating oyster mushrooms (Girmay et al., 2016, Mohamed et al., 2016, Tarko et al., 2018). Oyster mushroom (P. ostreatus) was grown on six different kinds of sawdust. Results revealed that treatment (T3) rain tree sawdust was found excellent substrate in all aspects from spawn running to yield (g), and biological efficiency (B.E%) (Bhattacharjya et al., 2014). Mainly agricultural residues and industrial raw material are used as substrates for the commercial cultivation of oyster mushrooms because they are available at low cost.
The present study aims to compare the various combinations of agricultural residues as substrates for the growth and production of oyster mushrooms (P. sapidus) to sort out the best substrates for its commercial cultivation in view the importance and benefits of mushrooms.
Materials and Methods
The methodology used in this study was standardized and followed Khan et al. (2017).
Experimental site and growth room conditions
The experiment was carried out in the mushroom growing room of Department of Plant Pathology, University of Agriculture Faisalabad (31.4504° N, 73.1350° E), Pakistan, during the growing season 208-19. The average room temperature and relative humidity were maintained at 18-25 °C and 70-80%, respectively.
Collection and preparation of the substrate
Maize residues (stalks, cobs, and leaves) and kikar tree (Vachellia nilotica) sawdust were used as substrates in different proportions. Maize residues were collected from the agronomic farm of the University of Agriculture Faisalabad. While kikar tree sawdust was collected from the local wood market of city Faisalabad. The substrate was prepared by a fermentation process using the methodology of Oseni et al. (2012). Maize crop was harvested at the time of maturity, chopped into pieces (2-3 cm) with the help of an electrical chopper, and dried under the shad two weeks before use. The fermentation of these materials (substrate) was done by soaking them separately in two drums containing sterilized distilled water (ddH20) for 24 h. After this, the soaked materials were spread on the polyethylene sheet on a cemented floor to remove the excess water. The material's moisture level was adjusted to 65-70% and pH 7.0 was maintained by adding the gypsum (@5% substrate dry weight) and thoroughly mixing it. The materials were then covered with a polyethylene sheet and airtight for anaerobic fermentation for seven days. After fermentation, the materials were mixed as per treatment ratios, and the following combination of the materials was used as substrates, as shown in Table 1.
| No. of Treatments | Combination Ratio |
|---|---|
| T1 | Sawdust 100% |
| T2 | Sawdust 75% + Maize residues 25% |
| T3 | Sawdust 50% + Maize residues 50 |
| T4 | Sawdust 25% + Maize residues 75% |
| T5 | Maize residues 100% |
Table 1. Combination of material used as a substrate for the cultivation of oyster mushroom (P. sapidus).
Filling and sterilization of the bags
After the fermentation, for each treatment, heat-tolerant polypropylene bags (8″×12″) were filled with 500 g of prepared substrate, and the mouth of the bags was closed with rubber bands. The bags were sterilized in an autoclave at 121 °C, 15 psi for 20 min and kept overnight in a hygienic room to cool's down the temperature of the bag at room temperature (Patel et al., 2012).
Collection and inoculation of spawn
Prepared spawn of oyster mushroom (P. sapidus) was collected from the mushroom lab, Department of Plant Pathology, University of Agriculture Faisalabad, and 30 g/bag of spawn were used for inoculation. For spawn inoculation, the mouths of bags were opened and thoroughly mixed in the upper 2-3 cm layer of the substrate, and bags were then placed in the growth room in complete darkness under controlled environmental conditions (Tirkey et al., 2017).
Data Recording
Data were recorded for the accomplishment of spawn running (no. of days) for 25%, 50%, 75%, and 100% development of mycelium, total mycelium growth to pinhead formation (no. of days), and pinhead formation to development of fruiting bodies (no. of days) as described by Khan et al. (2017).
Yield (g) and biological efficiency (%)
A total of three flushes were harvested at the maturity of oyster mushroom (P. sapidus), and data were collected in weight (grams) for each flush, and total yield (g) was collected by the average weight of three flushes. The biological efficiency of oyster mushroom (P. sapidus) was calculated as described by Ashraf et al. (2013), using the following formula:
Statistical Analysis
The whole experiment was carried out under a completely randomized design (CRD) with five treatments and seven analytical replications in each treatment, while each treatment was repeated three times. Data were statistically analyzed using the analysis of variance (ANOVA) by MSTAT-C (Version 5.4) software with a probability level of p≤0.05% to achieve the significance of the research (Jamil et al., 2019).
Results and Discussion
The accomplishment of spawn running in several days
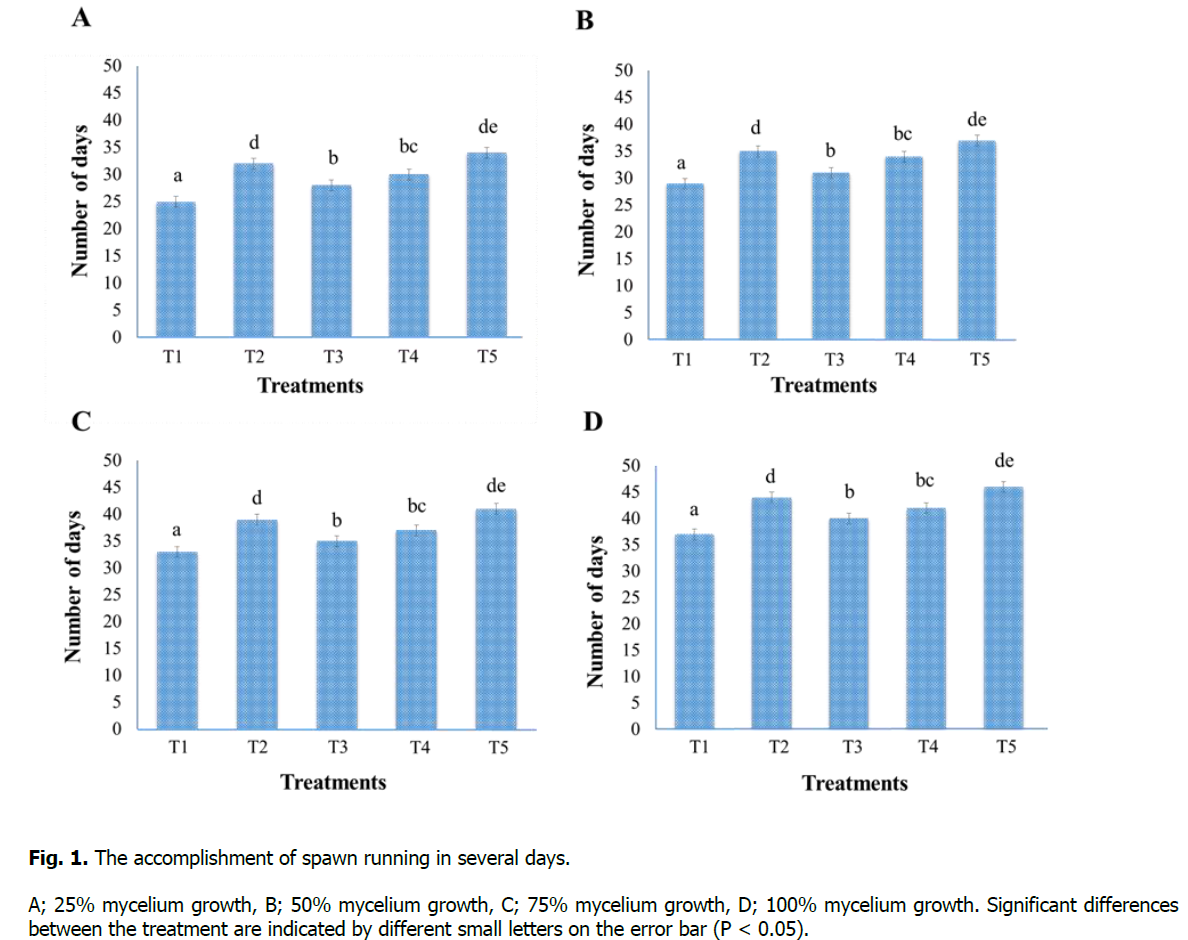
Data related to spawning running was taken in the number of days at 25%, 50%, 75%, and 100% mycelium growth and when bags turn white after the inoculation of spawn (Figure. 1). Results revealed that Treatment-T1 (sawdust 100%) has a significant difference for the accomplishment of spawn running in case of span (time) and took the minimum number of days for 25% (25 days), 50% (29 days), 75% (33 days), and 100% (37 days) mycelium growth. In comparison, the maximum number of days was taken by the Treatment-T5 (maize residues 100%) for 25% (34 days), 50% (37 days), 75% (41 days), and 100% (46 days) mycelium growth. The order of accomplishment of spawn running in the number of days is as follows: T1
Figure 1: The accomplishment of spawn running in several days.
A; 25% mycelium growth, B; 50% mycelium growth, C; 75% mycelium growth, D; 100% mycelium growth. Significant differences between the treatment are indicated by different small letters on the error bar (P < 0.05).
Jamil et al. (2019) studied the effect of various substrates on mycelium growth in periods (number of days). Three Pleurotus species P. sapidus, P. sajor-caju, and P. eryngii were examined, and results are similar to our findings, that P. sapidus took a minimum number of days (39), while P. sajor-caju and P. eryngii took a maximum number of days 67 and 77 respectively. The substrates rich in lignocellulosic and cellulosic substances were considered excellent substrates for timely and quick mycelium growth (Ullah et al., 2017). Jonathan et al. (2012) described that the spawn running is affected by the chemical composition of substrates and the presence of polyphenolic substances in these substrates.
Development of pinhead's and fruiting bodies
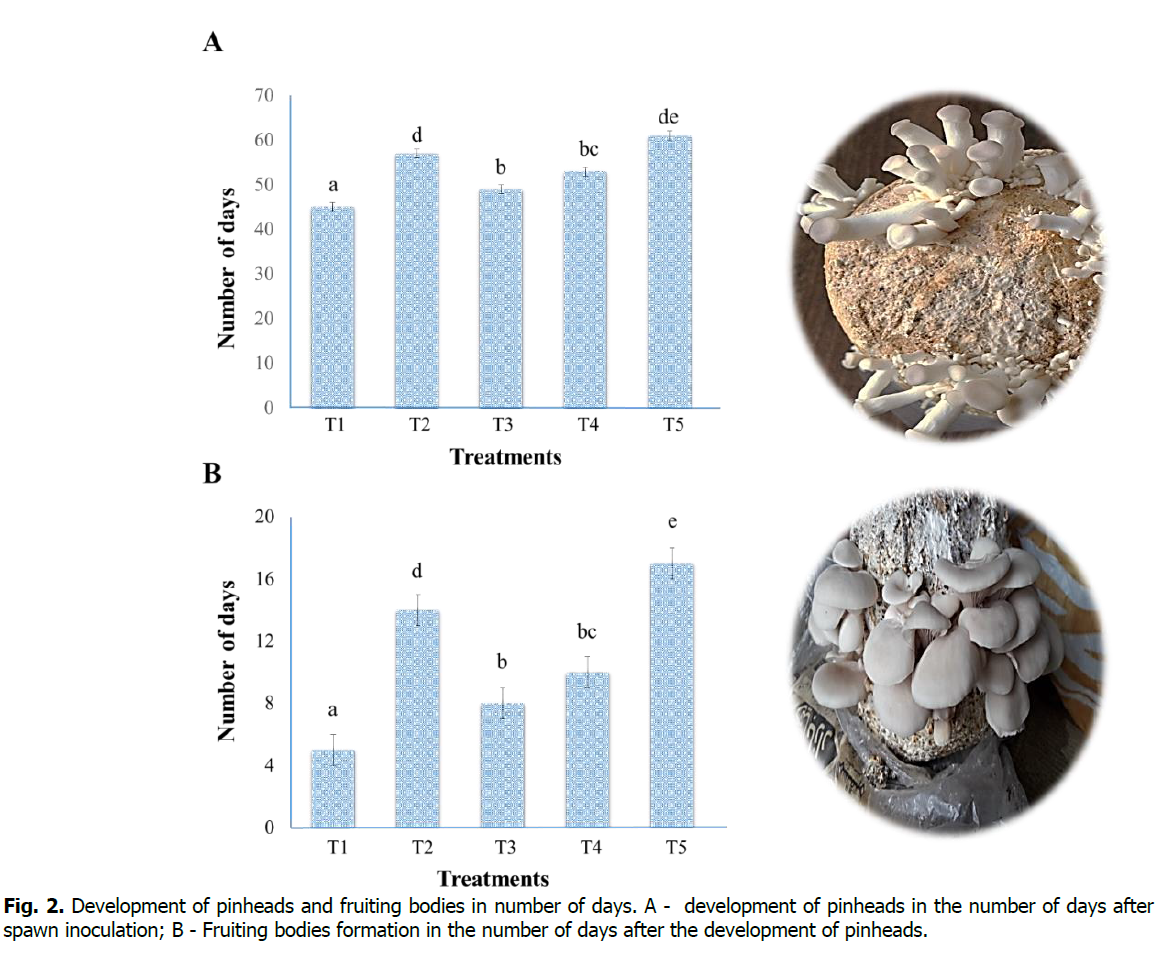
Data were recorded several days after the inoculation of spawn to the development of pinheads and for fruiting bodies formation after the development of pinheads on these substrates (Figure. 2). This study showed that Treatment-T1 (sawdust 100%) is found to be a good substrate in terms of pinheads and fruiting bodies development compared with other treatments. Treatment-T1 (sawdust 100%) took the least number of days (45 days) after the inoculation of spawn, which is only eight days after the 100% mycelium growth, and the minimum number of days (5 days) were recorded from pinhead's development to formation of fruiting bodies. However, Treatment-T5 (maize residues 100%) took the maximum number of days (61 days) after the spawn inoculation, which is 15 days after the 100% mycelium growth and thrice Treatment-T1. Similarly, the maximum number of days (17 days) for developing fruiting bodies after the pinhead formation. Our result revealed that Treatment-T5 (maize resides 100%) is not a suitable substrate for the quick and timely growth of pinhead's and fruiting bodies, and results are similar to the findings of Mshandete (2011).
Figure 2: Development of pinheads and fruiting bodies in number of days. A - development of pinheads in the number of days after spawn inoculation; B - Fruiting bodies formation in the number of days after the development of pinheads.
Significant differences in the development of pinheads and fruiting body formation between the treatments are indicated by different small letters on the error bar (P < 0.05).
Oei (2016) reported that factors such as; temperature, humidity, and quality of cellulose and lignin contents affect pinheads and fruiting bodies' development. Mshandete (2011) reported that the P. sapidus took (8-10 weeks) for pinhead development after spawn inoculation, similar to our results, but for fruiting bodies formation, it took (11 days); it may be due to temperature fluctuations during the fruiting period. Many studies have proven that on an excellent substrate which is rich in cellulose and lignin contents, pinheads developed within (7-8 ) days and fruiting bodies formed within (6-10) days (Girmay et al., 2016, Khan et al., 2017, Jamil et al., 2019). The substrates in carbon sources like; cellulose, hemicellulose, and lignin are considered suitable substrates for the cultivation of mushrooms (Al-Momany and Ananbeh, 2010, Alananbeh et al., 2014, Chang and Wasser, 2017).
Number of flushes and total yield
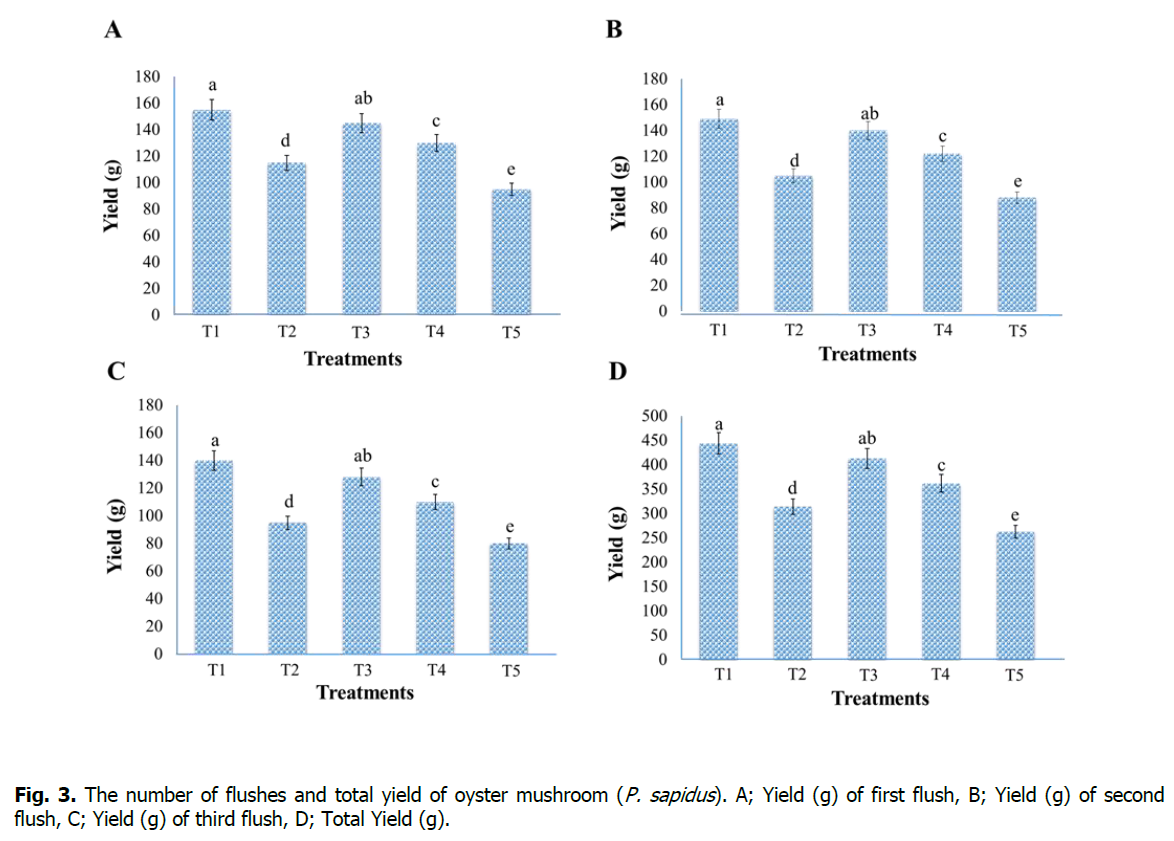
After the formation of fruiting bodies, harvesting of mushrooms was done at the maturity of each flush. A total of three flushes were harvested with an interval of seven days, and the yield of each flush was recorded in weight (g), while total yield was recorded by collecting the yield of all three flushes (Figure. 3). Results of this study revealed that the highest yield (g) was recorded for first flush (155 g), second flush (149 g), third flush (140 g), and total yield (444 g) in the case of Treatment-T1 (sawdust 100%). However, the lowest yield was recorded for first flush (95 g), second flush (88 g), third flush (80 g), and total yield (263 g) in case of Treatment-T5 (maize residues 100%). Our outcomes are related to previous studies' results (Mane et al., 2007, Isikhuemhen et al., 2009, Owaid et al., 2015), and it was observed that the highest yield was observed for the first flush, and with the passage of time the yield decrease gradually.
Figure 3: The number of flushes and total yield of oyster mushroom (P. sapidus). A; Yield (g) of first flush, B; Yield (g) of second flush, C; Yield (g) of third flush, D; Total Yield (g).
Significant differences between the first, second, and third flush yield and the total yield between the treatments are indicated by different small letters on the error bar (P < 0.05).
Isikhuemhen et al. (2009) proved that the highest yield of mushrooms depends upon indole acetic acid (IAA) and xylanase enzymes in the substrates. Dundar et al. (2008) studied the growth and yield performance of three Pleurotus sp. on different kinds of substrates. The result of their study showed that maximum yield (195 g) was recorded in the case of P. sapidus, while the other two species gave minimum yield. Similarly, Jamil et al. (2019) reported that P. sapidus has a good production ability and gave maximum yield compared with other Pleurotus sp. like; P. eryngii and P. sajor-caju. The result of this study is similar to our findings. Singh et al. (2012) stated that Pleurotus sp. can fix the nitrogen from the air, and temperature and light are the main factors which affect the yield of oyster mushrooms.
Biological efficiency
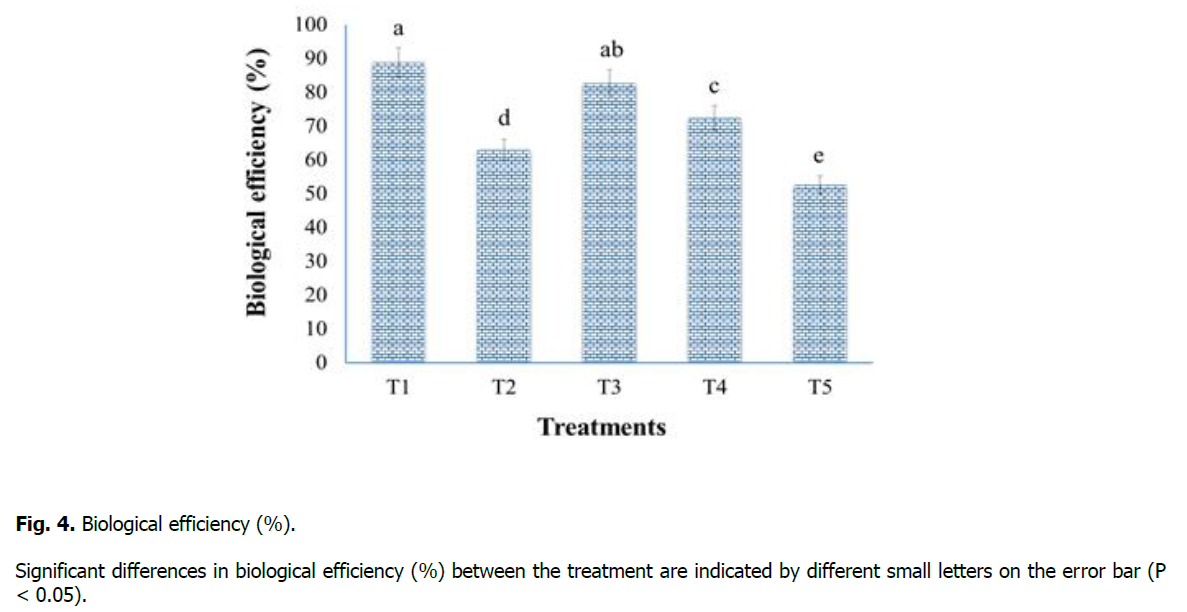
Biological efficiency (%) was recorded at the end of the experiment for substrate utilization ability of oyster mushroom (P. sapidus) by total yield (g) and substrate dry weight (g) ratio (Figure. 4). Results of our study showed that Treatment-T1 (sawdust 100%) has maximum biological efficiency (88.8%), while Treatment-T5 (maize residues 100%) has minimum biological efficiency (52.6%) and which is similar to the findings of Jamil et al. (2019) and Khan et al. (2021).
Figure 4: Biological efficiency (%).
Significant differences in biological efficiency (%) between the treatment are indicated by different small letters on the error bar (P < 0.05).
Conclusion
Commercial cultivation of oyster mushrooms (P. sapidus) depends on the availability of economical low-cost substrates and the substrates rich in carbon sources (lingo-cellulosic, cellulosic, and organic substances) are considered as excellent substrates of mushroom farming. In this study, we used maize residues (stalks, cobs, leaves) along with kikar tree (Vachellia nilotica) sawdust in different ratios as substrate, and we came to conclude that sawdust (100%) is found to be the best substrate for mushroom growth and gave maximum yield (g), and high biological efficiency (%). However, temperature, humidity, and light are the main factors which affect the growth of mushroom. Further research could be done to investigate the availability of fats, proteins, minerals, carbohydrates, and fiber contents in the oyster mushroom (P. sapidus) harvested on these substrates.
Author's Contribution
NAK and WA designed the study. UW and As. A performed the experiment and collect data. Ay. A, Aw. A, and BK analyze the data. WA and SM wrote the manuscript. All authors contributed to the final draft of the manuscript.
Conflict of Interest
The authors declared that they have no conflict of interest in this study.
References
Al-Momany, A. & Ananbeh, K. (2010). Conversion of agricultural wastes into value added product with high protein content by growing Pleurotus ostreatus. Survival and Sustainability. Springer.
Alananbeh, K. M., Bouqellah, N. A. & Al Kaff, N. S. J. S. J. O. B. S. (2014). Cultivation of oyster mushroom Pleurotus ostreatus on date-palm leaves mixed with other agro-wastes in Saudi Arabia. 21, 616-625.
Ashraf, J., Ali, M. A., Ahmad, W., Ayyub, C. M., Shafi, J. J. F. S. (2013). Effect of different substrate supplements on oyster mushroom (Pleurotus spp.) production. 1, 44-51.
Bhattacharjya, D. K., Paul, R. K., Miah, M. N., Ahmed, K. U. J. I. J. O. A. (2014). Effect of different saw dust substrates on the growth and yield of oyster mushroom (Pleurotus ostreatus). 7, 38-46.
Chang, S. T. & Wasser, S. P. (2017). The cultivation and environmental impact of mushrooms. Oxford Research Encyclopedia of Environmental Science.
Devi, M. K., Haobam, B., Devi, T. I., Singh, W. N. & Singh, W. J. J. S. E. R. (2015). Study of spawning response of Pleurotus sajor-caju in two different substrates (wheat and rice). 6, 1-3.
Dundar, A., Acay, H. & Yildiz, A. J. A. J. O. B. (2008). Yield performances and nutritional contents of three oyster mushroom species cultivated on wheat stalk. 7.
Girmay, Z., Gorems, W., Birhanu, G. & Zewdie, S. J. A. E. (2016). Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates. 6, 1-7.
Isikhuemhen, O. S., Mikiashvilli, N. A. J. J. O. I. M. (2009). Lignocellulolytic enzyme activity, substrate utilization, and mushroom yield by Pleurotus ostreatus cultivated on substrate containing anaerobic digester solids. 36, 1353-1362.
Jamil, F., Yaqoob, A., Mehmood, Z., Hamid, A., Shah, Z., Imtiaz, M., Jamil, N. & Ijaz, R. (2019). Comparative Study for Growth and Yield Performance of Oyster Mushroom (Pleurotus spp.) on Different Substrates under Temperate Condition.
Jonathan, S., Okon, C., Oyelakin, A., Oluranti, O. J. N. (2012). Nutritional values of oyster mushroom (Pleurotus ostreatus)(Jacq. Fr.) Kumm. cultivated on different agricultural wastes. 10, 186-191.
Khan, N. A., Ahmed, W., Khan, M. A., Yasin, O., Asad, S. & Munir, S. J. A. F. S. J. (2021). Effect of Different Kinds of Substrates on the Growth and Yield Performance of Pleurotus sapidus (Oyster Mushroom). 18-24.
Khan, N. A., Ahmed, W., Rehman, A., Jahangir, M. & Khan, B. J. P. J. B. (2017). Efficiency of oyster mushroom Pleurotus columbinus-P 8 using date palm leaves with combination of wheat straw and cotton waste for its yield improvement. 49, 2459-2464.
Kumar, V., Subha Chandra, M., Shancy, S., Sabnam, V. & Lamya, T. J. J. O. P. D. S. (2015). Cultivation of edible mushroom in India: Precautions, opportunities and challenges. 7, 409-413.
Mane, V. P., Patil, S. S., Syed, A. A. & Baig, M. M. V. J. J. O. Z. U. S. B. (2007). Bioconversion of low quality lignocellulosic agricultural waste into edible protein by Pleurotus sajor-caju (Fr.) Singer. 8, 745-751.
Mohamed, M. F., Refaei, E. F., Abdalla, M. M. & Abdelgalil, S. H. J. I. J. O. R. O. O. W. I. A. (2016). Fruiting bodies yield of Oyster Mushroom (Pleurotus Columbinus) As Affected By Different Portions Of Compost In The Substrate. 5, 281-288.
MSHANDETE, A. M. J. I. J. O. R. I. B. S. (2011). Cultivation of Pleurotus HK-37 and Pleurotus sapidus (oyster mushrooms) on cattail weed (Typha domingesis) substrate in Tanzania. 1, 135-144.
Oei, P. (2016). Mushroom Cultivation IV: Appropriate Technology for Mushroom Growers, ECO Consult Foundation.
Oseni, T. O., Dlamini, S. O., Earnshaw, D. M., T Masarirambi, M. J. I. J. O. A. & Biology (2012). Effect of substrate pre-treatment methods on oyster mushroom (Pleurotus ostreatus) production. 14.
Owaid, M. N., Al-Saeedi, S. S. S., Sabaratnam, V., Al-Assaffii, I. A. A. & Raman, J. J. J. J. O. A. I. B. (2015). Growth performance and cultivation of four oyster mushroom species on sawdust and rice bran substrates. 4.
Patel, Y., Naraian, R., Singh, V. J. W. J. O. F. & Biology, P. (2012). Medicinal properties of Pleurotus species (oyster mushroom): a review. 3, 1-12.
Pekşen, A. & Küçükomuzlu, B. J. P. J. O. B. S. (2004). Yield potential and quality of some Pleurotus species grown in substrates containing hazelnut husk. 7, 768-771.
Randive, S. D. J. A. I. A. S. R. (2012). Cultivation and study of growth of oyster mushroom on different agricultural waste substrate and its nutrient analysis. 3, 1938-1949.
Sharma, V., Annepu, S. K., Gautam, Y., Singh, M. & Kamal, S. J. M. R. (2017). Status of mushroom production in India. 26, 111-120.
Shirur, M. & Shivalingegowda, N. J. M. J. A. S. (2015). Mushroom marketing channels and consumer behaviour: A critical analysis. 49, 390-393.
Singh, M., Pandey, A., Vishwakarma, S., Srivastava, A., Pandey, V. J. C. & Biology, M. (2012). Extracellular xylanase production by Pleurotus species on lignocellulosic wastes under in vivo condition using novel pretreatment. 58, 170-173.
Solovyev, N., Prakash, N. T., Bhatia, P., Prakash, R., Drobyshev, E., Michalke, B. J. J. O. T. E. I. M. (2018). Selenium-rich mushrooms cultivation on a wheat straw substrate from seleniferous area in Punjab, India. 50, 362-366.
Tarko, D. B., Sirna, A. M. J. J. O. A. B. (2018). Substrate optimization for cultivation of Pleurotus ostreatus on lignocellulosic wastes (coffee, sawdust, and sugarcane bagasse) in Mizan–Tepi University, Tepi Campus, Tepi Town. 6, 14-20.
THAKUR, M. J. I. P. (2020). Advances in mushroom production: key to food, nutritional and employment security: A review. 73, 377-395.
Tirkey, V. J., Simon, S., Lal, A. A. J. J. O. P. (2017). Efficacy of different substrates on the growth, yield and nutritional composition of oyster mushroom-Pleurotus florida (Mont.) Singer. 6, 1097-1100.
Ullah, T. S., Firdous, S. S., Mehmood, A., Shaheen, H. & Dar, M. E. U. I. J. I. J. O. M. M. (2017). Ethnomycological and nutritional analyses of some wild edible mushrooms from Western Himalayas, Azad Jammu and Kashmir (Pakistan). 19.
Varghese, B. P. & Amritkumar, P. J. I. J. S. R. I. B. S. V. (2020). Comparative Study on Cultivation of Oyster Mushrooms using Nutrition Enhancing Substrates. 7.
Author Info
A. K. Nasir1, T. W. Urwa1, A. Waqar1*, A. Ayesha2, A. Awais3, A. Asma1, K. Babar1 and M. Shahzad22College of Plant Protection, Yunnan Agricultural University, Kunming, 650201, Yunnan, China
3Department of Mathematics, Maharishi University of Management and Technology, 54000, Lahore, Pakistan
Citation: Nasir, A. K., Urwa, T. W., Waqar, A., Ayesha, A., Awais, A., Asma, A., Babar, K., Shahzad, M. (2021). Effect of Maize residues (stalks, cobs, leaves) and sawdust substrates on the growth and yield of oyster mushroom (Pleurotus spaidus). Ukrainian Journal of Ecology, 11 (2), 1-7.
Received: 08-Feb-2021 Accepted: 08-Mar-2021 Published: 31-Mar-2021, DOI: 10.15421/2021_68
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.