Research - (2021) Volume 0, Issue 0
Effect of parasitic copepods on the growth of Abramis brama fish from Beni-Haroun dam of Mila city (Northeast Algeria)
B. Houda1,2*, S. Fatiha1,2, K. Nouha1,2 and B. Chahinaiz2Abstract
The growth parameters are valuable data to provide a good understanding on the general biology and the population dynamics of fishes, as well as enable to establish the mathematical models to compare the growth curves, and subsequently to perform the demographic analyses.
Thus, the present work was undertaken to study the effect of two parasitic copepods, namely Ergasilus sieboldi and Ergasilus briani on the growth of 141 fish individuals of Abramis brama species from Beni-Haroun dam of Mila city (northeast Algeria). Herein, the study was conducted on 141 fish individuals during the period July 2015-October 2016. The age of fishes was determined by scalimetric method since the fish sex was macroscopically determined where the number of females was found very higher (97) than that of males (44).
Moreover, the fish growth study was performed according to the mathematical method of Von Bertalanffy (1938). The determined growth parameters in Abramis brama fish affected by the two parasitic copepods showed that the non-parasitized host fishes present the following growth values: L∞=40 cm; K=0,30; t0=-0,29; Ø’=2,68, while the growth values of the parasitized host fishes are L∞=30 05 cm; K=0,31; t0=-0,27; Ø’=2,48. Hence, these parasites slow down the absolute growth in length of the parasitized fishes, which in turn suffer the decline in their K condition index (K=0,10) compared to non-parasitized fishes (K=0,22). Further, the evolution of the studied total fish weight in relation to their size exhibits a negative (minor) allometry (b<3) for the non-parasitized and parasitized specimens (without distinction between both sexes).
Keywords
Abramis brama, Parasitic copepods, Von Bertallanffy model, K condition index, Beni Haroun dam.
Introduction
The living organisms can grow from birth by an increase in size, occurring through differences in rates of some stages of biological and individual processes, including sexual differentiation. Whilst, this phenomenon can slightly slow down in a given moment, before the death of the organism and up, even to the complete standstill in some cases. Thus, the growth term includes some different concepts from the cellular processes to the growth of populations.
Further, Copepods are crustacean parasites whose effect leads to serious economic losses, and indeed in some cases, the impact of the parasitism of the dynamic of fish populations is significant, and consequently, it induces great economic losses, especially in fish breeding (Bragoni et al, 1983). Some authors have proved that the parasitized fishes undergo a decline in the condition index, and a reduced growth (Romestand et Trilles, 1979; Adlard et Lester, 1994; Ramdane, 2009), in addition to reproduction disorders and a reduced lifetime (Adlard et Lester, 1994). Also, some other authors have reported that Ergasilidae infesting fish gills would cause loss of fish populations and a significant loss of weight, leading thus to reduce the fish productivity (Dogiel et al, 1958). Meanwhile, several authors have reported that the attack by Ergasilus sieboldi is common in many host species of various freshwater bodies (Boucenna et al, 2015; Berrouketal, 2018). Interestingly, the literature has mentioned several growth models, among which those of natural populations applied to breeding populations since the most used growth is that of Von Bertalanffy (1938) using the adjustment parameters which can be determined by different statistical methods or by computer software programs (Saila et al, 1988). This model is used to determine the growth of different species and defined as a standard model enabling the comparison of the growth between species and populations (Dall et al, 1990; Wahle and Fogarty, 2006).
Materials and Methods
Description of the study area
Beni-Haroun dam is a large strategic hydraulic complex located in Mila city (northeast Algeria) and monitors the basin waters of the Wadi-Kebir Rhumel river. The dam is the head of a large hydraulic transfer system, including a huge pumping station with a water flow of 21 m3|s on a drop of 700 m, and whose impact would affect five Eastern Algerian cities (Mila, Constantine, Oum el-Bouaghi, Batna, and Khenchela). The dam has a capacity of 960 million cubic meters for a dike of 710 m in length and 120 m in height, and a total drained surface of 7725 Km2. The dike is located narrowing of limestone gorges, where the geology becomes complex and highly sheared (Marmi et al., 2008). This hydrographic entity is composed of four sub-watersheds (Table 1), and is naturally surrounded by the following basins (Kerdoud, 2006):
• From the North-west and East, the middle-east coastal basin of Constantine city.
• From the South, the basin of Constantine highlands.
• From the West, the basins of the Agerois-Hodna-Soummam.
• From the East, the Seybouse basin.
Methods of sampling, identification, and dissection of host fishes
Herein, 141 fish individuals of Abramis brama were seasonally sampled during 2015-2016. The obtained fishes were immediately transported to the laboratory for the specific identification based on the criteria and the nomenclature proposed by Leveque et al. (1990). Also, fishes were weighed and measured (total length) before being dissected, and after death, gill arches (dorsal and ventral gills) were carefully removed by two incisions and then kept in pillboxes containing ethanol at 70% concentration.
Collection and identification of parasites
The extraction and the observation of parasites were conducted when each pillbox containing the gill arches is poured into a Petri dish and then subjected to a precise binocular observation. The parasites were afterward collected using a fine brush, and placed into new pillboxes containing 5% formalin. Each pillbox is labeled by the observer's initials, the date of sampling, and the number of the sample, meanwhile, a sampling card is filled in for each fish with fish’s name, biometric parameters, and the identified parasite species. (Berrouk, 2019)
Determination of the parasite species
The preserved samples in the pillboxes were re-examined, and every identified parasite was the subject of a deep study for the species determination (Fig. 1).
Fig 1. Geographical location of Beni-Haroun dam of Mila city. Algeria.
Treatment of data
Epidemiological indices: The epidemiological indices examined in this study are prevalence, average parasitic intensity, and parasitic abundance. These parameters are defined according to Margolis et al. (1982) and Bush et al. (1997) as follow:
• Prevalence (P%) is the percentage ratio (%) of the number of infected host fish (N) with a given parasite species divided by the number of the examined fishes (H).
P(%)=N/H × 100
• Average parasitic intensity (I)
It corresponds to the ratio of the number of total individuals of the parasite species (n) in a sample of infected hosts in the sample and thus is the mean number of individuals of a parasite species divided by the number of parasite-infected hosts in the sample.
IM=n/N
• Abondance parasitaire (A)
It is the ratio of the number of total individuals of the parasite species (n) in a sample of hosts divided by the number of total fish (H) of the sample and thus is the mean number of parasite species individuals per the examined fish.
AM=n/H
Where “n”, is the number of parasites, “N” is the number of infected hosts, "H” is the number of examined fishes, “P” is the prevalence, “IM” is average intensity, and “AM” is mean abundance.
This study aims to identify the gill tissues of crustacean ectoparasites harvested from Abramis brama fish through observations of morpho-anatomical features, and to determine the parasitic indices according to sex, size, seasons and micro-habitats (right and left gills).
Physicochemical analysis of water: The physicochemical parameters were monitored with accordance with the stuff of the National Agency for Hydraulic Resources (NAHR), who have ensured the monitoring of the quality of water dam intended to supply the drinking water from a station at the level of the dam, providing a restricted access for the agency staff. Also, the physicochemical parameters collected monthly throughout the study period (2015-2016), considered as about one ecological year are as follow temperature (T), power of hydrogen (pH), dissolved oxygen (O2), nitrates (NO3-), nitrites (NO2), ammonium (NH4), organic matter levels (OM), phosphate (PO4), biological oxygen demand (BO5D), chemical oxygen demand (COD) and dry matter residue (DMR).
Statistical analysis of data
The statistical analysis of data was performed by using Statistica software for Windows (Version 8.0). Bivariate statistical comparison of data was based on the determination of the correlation coefficient of Pearson. A pairwise correlation was conducted to highlight whatever relationship between the intensity of crustacean parasites collected from Abramis brama and the nine physicochemical parameters of Beni-Haroun dam water. The existence of a significant correlation was evidenced by determining the P values of each determined r value.
Of note,
p >0.05 ⇒ No correlation.
p ≤ 0.05 ⇒ Significant correlation ⇒ *
p ≤ 0,01 ⇒ Highly significant correlation ⇒ **
p ≤ 0,001⇒ Very highly significant correlation ⇒ ***
Study of fish growth
Determination of age and growth of parasitized and non-parasitized fishes: We have estimated the growth of parasitized and non-parasitized host fishes of both sexes, and we have tested the effect of parasitism on the growth of fishes. The fish age was determined based on scales taken from the laterodorsal parts, showing earlier scales (Boet and Louarn, 1985). The removed scales were cleaned by running water, rubbed between thumb and forefinger to rid them from the covering tissue fragments and mucus, and then sorted under a binocular magnifying glass (Bouhbouh, 2002).
Von Bertalanffy growth model: Here, the growth model was developed by Von Bertalanffy (1938), and is based on two different processes; the first one is anabolism process increasing the animal weight since the second one is catabolism process decreasing the animal weight. These two processes act simultaneously and continuously throughout the life of individuals. At a point in time, the difference between the two processes defines the rate of change in the animal weight at this time point, and hence this is evidenced by an equation, in which the anabolism rate is considered as proportional with the absorbing surfaces, and the catabolism rate is proportional with weight. According to Von Bertalanffy (1938), the linear growth equation is given as follows:
Lt=L∞ [1-e–k(t-tо)]
The following growth parameters; L∞, K, and t0 characterize the conducted adjustment, and the definitions of these parameters as provided recently are as follow:
L (t): Fish length studied in function of time t.
L∞: is asymptotic size known also as the asymptotic length of L∞ when t tends toward infinity, but it must not be confused with the maximum length. It is also defined as the mean size reached by the fish which could survive and grow indefinitely (Pauly and Moreau, 1997). The estimation of the asymptotic length L∞ is performed according to the method of Pauly (1985) using the maximum length Lmax observed in a species. The formula is as follows:
L∞=Lmax/0.95
Lmax: Size of the biggest fish measured in the sample.
K: Growth coefficient expressing the protein degradation or synthesis in the fish body, it expresses the biotic and abiotic factors limiting the availability of oxygen, and characterizes the growth speed, by which the fish grows toward the asymptotic length. Further, the estimation of “K” coefficient appealed to the method of Pauly and Munro. (1985) based on the growth speed value determined from the values of L∞ and K according to the following relation:
Log10 (-t0)=-0.3922-0.2752Log10 L∞-1.038 Log10K
The comparison of the growth parameters of the same or different stock of the same species was tested by Phi-prime (Ø') described by the following formula:
Ø'=log K+2log L
According to Pauly (1979), the Phi-prime values within the neighboring taxa are very similar and have normal and narrow distributions (Sparre et Venema, 1996). Also, the growth parameters from Von Bertalanffy equation (L∞, K et t0) were determined by the method of Ford Walford (1946) using Statistica software (Version 8.0).
Van Bertalanffy (1938) relative weight growth model: This model promotes to estimate the fish weight from the size or vice versa (Beyer, 1991), and the weight from age (Petrakis and Stergiou, 1995), as well as it enables to express the equation of linear and weight growth, and consequently this relation is used to describe the fish species forms, and to know the overweight and evolution of fishes during a given period. The relative growth is applied in various biological, physiological, and ecological fields. Also, it enables to check the existence of a correlation relating weight and size of the fish and even leads to improve this relationship. Whereas the fish weight becomes a cube proportional to its length when fish keeps the same general form and weight during its life.
Pt=a. Ltb
Where “Pt” is the total weight (g); “Lt” is the total length (cm), “a” is a constant value, and “b” is the allometric coefficient.
According to Gayanilo et al. (1996), this equation can be can be linearized by the logarithmic transformation of the data to result in:
Log Pt=b Log Lt+ Log a
This transformation reduces the viability and homogenizes the two variables (total and gutted weight and total length), enabling thus to determine the least squared values of the parameters: “b” slope and “a” (points ordered from origin) of this regression line.
Where “P” is the weight of specimens (g), “a” is the proportionality constant, Lt is the allometric coefficient, providing information about the growth weight.
The “b” value is statistically compared at b0=3, and P=0,05 using the t-Student test (Dagnelie, 1975).
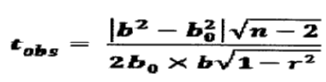
n: Effectif
b: Slope (b0=3)
r: Correlation coefficient.
Noteworthy, three cases can be displayed in relation to the slope “b” of the regression line as follow:
• b=3 : Weight changes proportionally with the cube of the length, and thus the relation is considered perfect isometry.
• b# 3 : The growth is allometric.
• b >3 : The weight grows more rapidly as compared to the cube length, and thus the allometry is major (positive).
• b <3 : The weight grows less rapidly as compared to the cube length, and thus the allometry is negative (minor).
Von Bertalanffy (1938) absolute growth weight model: The linear growth equation and size-weight relationship enable us to establish the growth equation in the weight of Von Bertalanffy or in weight growth. The descriptive model of Von Bertalanffy (1938) weight growth is given by the following relation.
Pt=P ∞ (1-e –k(t-tо)]
Where “Pt” is the total weight in time t, “P∞ “ is the asymptotic weight corresponding to L∞, “b” is the allometric coefficient, “K” and t0 are Von Bertalanffy equation parameters.
Fulton’s condition index (K)
The condition index “K” characterizes excess weight, nutritional status, and energy reserves of an individual. This morphometric index assumes that the heavier fish for a given length is in better condition. The condition factor K was calculated according to Fulton formula:
K=(P) /Ltb.
Where “b” is the allometry coefficient.
Results
Identification of crustacean parasites collected from Abramis brama
The examination of 141 gills of Abramis brama fishes obtained from Beni-Haroun dam during 2015-2016 enabled us to collect eight copepod parasites. Further, the observation of morpho-anatomical criteria of the collected parasites enabled us to inventory two parasite species, namely Ergasilus sieboldi and Ergasilus briani related to genus Ergasilus and Ergasilidae family (Fig. 2).
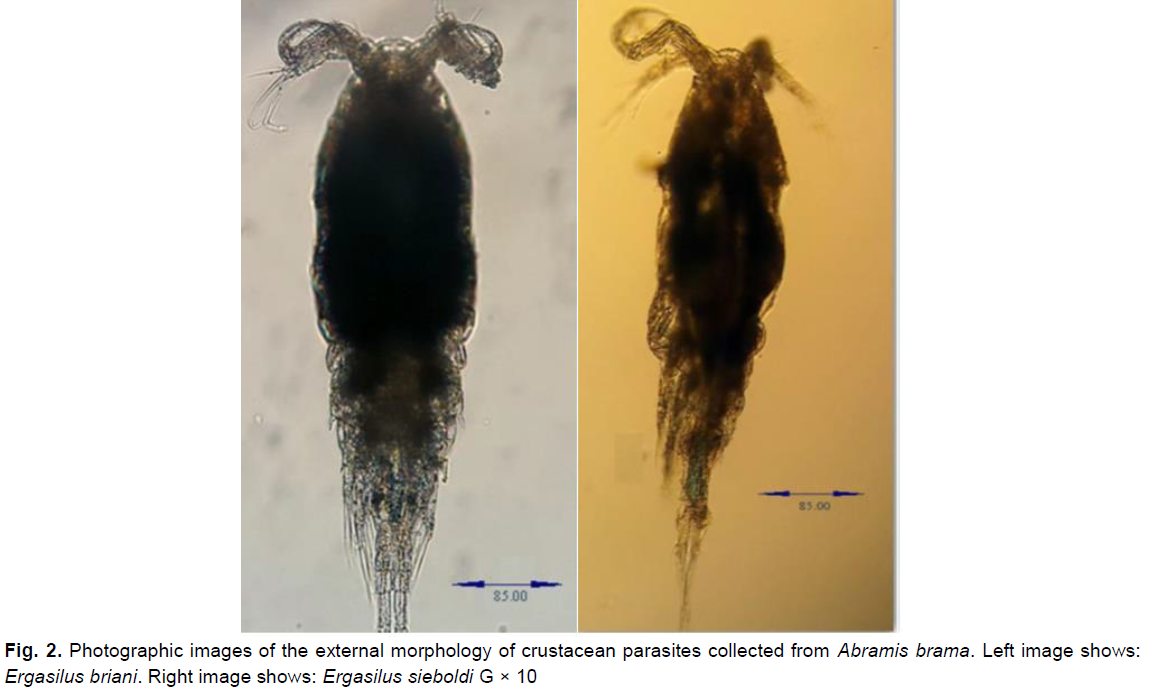
Fig 2. Photographic images of the external morphology of crustacean parasites collected from Abramis brama. Left image shows: Ergasilus briani. Right image shows: Ergasilus sieboldi G × 10
Epidemiological indices
As shown in Table 1, the epidemiological indices, including abundance, intensity, and prevalence are weak according to the number of infested fishes (8 fishes) and the number of collected parasites (8 parasites). Also, the highest prevalence values were noticed in autumn, since the parasitic intensity values were equal during the four seasons.
| Variables | Winter | Spring | Summer | Autumn | Total |
|---|---|---|---|---|---|
| NEF | 41 | 22 | 46 | 32 | 141 |
| NIF | 0 | 1 | 3 | 4 | 8 |
| NP | 0 | 1 | 3 | 4 | 8 |
| P% | 0 | 4.54 | 6.38 | 12.5 | 23.42 |
| I | 0 | 1 | 1 | 1 | 3 |
| A | 0 | 0.04 | 0.06 | 0.12 | 0.22 |
Table 1. Distribution of the parasitic indices according to the four seasons
Table 2 shows that the small-sized fishes are the most infested, where the values of prevalence and intensity are higher.
| Variables | [15-25] | [25-35] | Total |
|---|---|---|---|
| NEF | 73 | 68 | 141 |
| NIF | 6 | 2 | 8 |
| NP | 6 | 2 | 8 |
| P% | 8.22 | 2.89 | 5.63 |
| I | 1 | 1 | 1 |
| A | 0.08 | 0.03 | 0.05 |
Table 2. Distribution of the parasitic indices according to the size classes.
As indicated in Table 3, the values of prevalence and abundance are higher in females than those in males.
| Variables | Males | Females |
|---|---|---|
| NEF | 97 | 44 |
| NIF | 4 | 4 |
| NP | 4 | 4 |
| P% | 4.08 | 9.09 |
| I | 1 | 1 |
| A | 0.04 | 0.09 |
Table 3. Distribution of the parasitic indices according to both sexes.
In Table 4, the number of the obtained parasites is higher in right gills than that of the left gills where the highest values of the parasitic indices are observed also in the right gills.
| Variables | Right Gill | Left Gill |
|---|---|---|
| NEF | 141 | 141 |
| NIF | 5 | 3 |
| NP | 5 | 3 |
| P% | 3.52 | 2.11 |
| I | 1 | 1 |
| A | 0.03 | 0.02 |
Table 4. Distribution of the parasitic indices according to both fish gills (right and left gills).
Relationship between physicochemical parameters and the parasitic intensity of copepods collected from Abramis brama.
A correlation analysis was performed to demonstrate the possible correlation between physicochemical parameters (abiotic) of Beni-Haroun dam waters and the parasitic intensity of crustaceans collected from Abramis brama (Table 5).
| Parametres | pH | O2 mg/l | DMR | NO3-mg/l | NH4+ mg/l | PO4¯3 mg/l | BDO | COD | NO2- mg/l |
|---|---|---|---|---|---|---|---|---|---|
| r=0.16 p=0,72 |
r=0.68 p=0.09 |
r=0.18 p=0.69 |
r=0.34 p=0.45 |
r=0.38 p=0.40 |
r=0.28 p=0.53 |
r=0.76 p=0.04* |
r=0.60 p=0.14 |
r=0.23 p=0.61 |
Table 5. Correlation analysis between the parasitic intensity in Abramis brama and various physicochemical parameters of Beni-Haroun dam.
Age study of unparasitized and parasitized Abramis brama (Fig. 3)
Growth study of Abramis brama affected by copepods parasites Linear growth
Table 6 shows the growth parameters (L∞, K, t0, and performance index Ø) of parasitized and non-parasitized Abramis brama fish from Beni-Haroun dam according to the model and the equation of Von Bertalanffy (1938) linear growth. The “K” values of parasitized breams are higher than those of non-parasitized breams. Whilst, the asymptotic size (L∞) obtained for parasitized and non-parasitized breams is, respectively 40 cm and 29,47 cm with difference of 10,53 cm. These results highlight a differential growth between the parasitized and non-parasitized breams in favor of parasitized specimens (Tables 7-10) (Fig. 4).
Relative weight growth; Size-Weight relationship
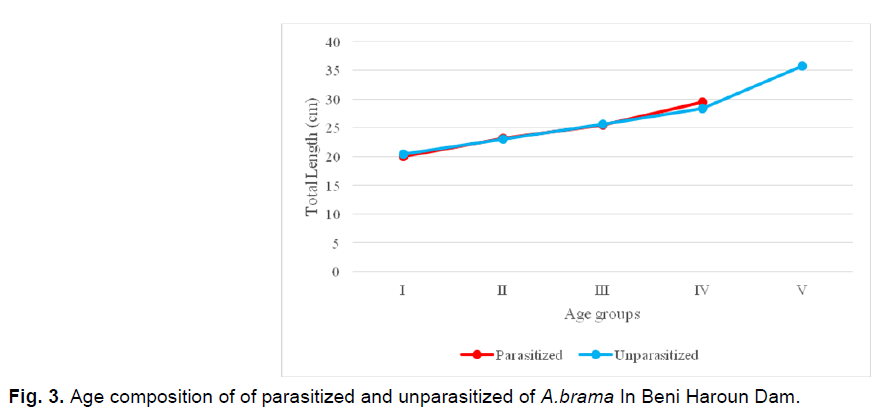
Fig 3. Age composition of of parasitized and unparasitized of A.brama In Beni Haroun Dam.

Fig 4. Relationship size-weight: A-Unparazited, B-Parasited specimens of A. brama.
| Parameter | N | L∞ cm | K(year-1) | t0 (year) | Ø’ | Lmin_Lmax cm |
|---|---|---|---|---|---|---|
| Non-parasitized A. brama | 133 | 40 | 0.30 | -0.29 | 2.68 | 19.5-38 |
| Parasitized A. brama | 8 | 31.05 | 0.31 | -0.27 | 2.48 | 19.5-29.5 |
Table 6. Von Bertalanffy linear growth equation parameters of parasitized and non-parasitized Abramis brama specimens.
| Parameter | Equation |
|---|---|
| Non-parasitized brème | Lt=40[1-e⁻0’30(t+0’29)] |
| Parasitized brème | Lt=31,05[1-e⁻0’31(t+0’27)] |
Table 7. Von Bertalanffy linear growth equation of parasitized and non-parasitized Abramis brama fishes from Beni-Haroun dam.
| Parameter | n | a | b | r2 | t cal | significance | GT | W=a*TLb |
|---|---|---|---|---|---|---|---|---|
| Unparasitized brème | 133 | 0.22 | 2.01 | 0.66 | 7.86 | + | A- | W=0.22TL 2.01 |
| Parasitized brème | 8 | 0.10 | 2.23 | 0.91 | 2.70 | + | A- | W=0.10TL 2.23 |
Table 8. Parameters of size-weight relationship for parasitized and non-parasitized Abramis brama fishes.
| Parameter | Equation |
|---|---|
| Non-parasitized A. brama | Pt=-0,7 Lt2’04 |
| Parasitized A. brama | Pt=-1,29 Lt2’44 |
Table 9. Von Bertalanffy linear growth equation of parasitized and non-parasitized Abramis brama fishes from Beni-Haroun dam.
| Size/total weight allometric test | b | Isometric growth |
|---|---|---|
| Non-parasitized A. brama | 2.04 | Negative (minor) allometric growth |
| Parasitized A. brama | 2.23 | Negative (minor) allometric growth |
Table 10. Size/total weight allometric test of parasitized and non-parasitized Abramis brama fishes from Beni-Haroun dam.
Condition indexThe effect of parasitism of bream population shows that the recorded “K” values in the non-parasitized specimens (K=0.91) are higher than those observed in the parasitized specimens (K=0.87) (Table 11).
| Parameters | N | K (g.cm-3) |
|---|---|---|
| Non-parasitized A. brama | 133 | 0.22 |
| Parasitized A. brama | 8 | 0.10 |
Table 11. Condition index of parasitized and non-parasitized Abramis brama fishes from Béni-Haroun dam.
Discussion
Epidemiological study
The findings of the present investigation confirm the presence of parasitism in Beni-Haroun dam although of the quite low infestation rates of all copepod parasites. Hence, the examination of 141 Abramis brama fish individualsenabled us to collect only eight parasite individuals attached to two fish species (Ergasilus sieboldi and Ergasilus briani). In this regard, Boucenna (2017) has found no parasitic infestation in Abramis brama from two dams of North-Eastern Algeria (Foum-El-Khonga and Ain-Eldalia), while Berrouk (2019) has noticed the presence of Ergasilus sieboldi and Ergasilus briani in Luciobarbus callensis, Carassuis carassuis and Cyprinus carpio from Beni-Haroun dam. Further, Ergasilus sp is a worldwide parasite whose parasitic infestation leads to a major parasitic disease for marine organisms, as well as the parasite species has no specificity for host and can infest the majority of freshwater fishes (Piasecki et al (2004). Nevertheless, the results of the present study showed the highest values of parasitic intensity and prevalence in Abramis brama during autumn, followed by summer and spring, since no parasitic infestation was observed in winter. Benmansour (2001) has reported the important role of seasons in the development and the abundance of the copepod parasites, and also according to Koskivaara et al, (1991), the water temperature is generally considered as an important factor determining the presence and the abundance of parasites. Regarding the parasitism study in function of Abramis brama sex, no significant difference in the prevalence of the collected copepod parasite was noticed between fish sexes, suggesting thus that both sexes are infested in the same manner. In addition, the absence of the fish sex effect on the parasitic infestation in the monogenic gills of Hemichromis fasciatus has been already reported by Bilong-Bilong (1995). The study of parasitism as a function of size classes in Abramis brama brought to light that the small-sized specimens show generally the highest values of the parasitic indices. Furthermore, the study of Boucenna (2017) conducted on Carassuis carassuis from Ain Dalia dam (northeast Algeria) has indicated that medium-sized fish classes exhibit the highest parasitic infestation rates. However, Ibrahimi (2012), Stavrescu-Bedivam et al (2014), Boucenna et al (2015) have found the big-sized fishes are the most infested by copepod parasites, meanwhile Poulin (2000) has claimed that this model can not be generalized, where some quantitative and qualitative differences are found in the study of the size class fishes. On top of that, the distribution of the parasitic indices of copepod parasites collected from Abramis brama according to the microhabitat is slightly increased in the right gills compared to the left gills. Boucenna (2017) has also noticed significant differences between the microhabitat and the parasitic copepod infestation rates in Luciobarbus callensis from Foum-El Kahnga dam (Northeast Algeria). Similarly, Berrouk (2019) has found significant differences between microhabitat and parasitic copepod infestation rates in Cyprinus carpio, Luciobarbus callensis, and Carassuis carassuis from Beni-Haroun dam.
Effect of abiotic parameters on the parasitic infestation
According to Marcogliese (2005), parasitism must be studied with respect not only to the undesirable environmental conditions but also to the diversity changes, prevalence, richness, and abundance of parasitism in response to the environmental stress factors. Besides, the ectoparasites are in constant contact with water, and this suggests that the bad water quality may adversely affect the parasite diversity in the greatest extent (Avenant Oldewage 2001). Moreover, the use of Pearson correlation coefficient has shown that the intensity of crustacean parasites collected from Abramis brama is negatively correlated with the Biological Oxygen Demand (BOD; r=0,76, p=0.046*), since the other physicochemical parameters (pH, O2, DMR, etc.) of Beni-Haroun dam show a correlation with a parasitic infestation, but not significant. In this context, Singhal et al. (1986) have found a positive correlation between the parasitic intensity of Gyrodactylus sp (monogenic) and pH values, suggesting hence that these parasites may support the low pH values with a considerable adaptation to support these conditions. Accordingly, Khidr (2012) have reported that the average parasitic intensity of Microcotyloides sp was positively correlated with water temperature and pH, and negatively correlated with water salinity, while the dissolved oxygen has shown no effect on the intensity of this parasite. Previous studies (Cone et al., 1993; Macrocogliese and Cone, 1996) have reported a decrease in the species richness of the assembly of parasite communities hosted by the yellow eels sampled from the sites of low pH in only one watershed. On the other hand, Brahmia et al, (2016) have shown that the abundance of B. acheilognathi was correlated with the increase in the values of dissolved oxygen. Also, the study of Al-Niaeem et al, (2015) investigating the effect of the water quality on infested fishes by copepods from three stations of Basra city (Iraq) has reported that the composition of parasite species and richness of parasites infecting freshwater fishes are influenced by the environmental factors. These results suggest also that the number of copepod species decreases in disturbed environments because they are directly exposed to water quality effect. Some previous studies (Oros and Hanzelová 2009; Madanire-Moyo and Barson, 2010) have shown the existence of a close and very sensitive relationship between the environmental conditions and parasitism. As reported by Esch et al, (1993), the environmental factors are important in the recruitment, transmission, colonization, fertility, and survival of adult and larval parasites.
Study of the growth of Abramis brama under the effect of two copepod parasites
The effect of crustacean parasites on the growth of Abramis brama in Beni-Haroun dam was studied according to Von Bertalanffy (1938) growth model comparing the parasitized and non-parasitized specimens. The obtained results indicate that the copepod parasites result in a decrease of the absolute growth and the asymptotic size (L) of Abramis brama. These findings are in line with those reported by Cassier et al. (1998) who have observed a decrease in the infested mullet by Ergasilus lizae, and have proved that the fish weight loss due to crustacean parasite effects is frequently observed, and here, these authors have recorded several weight loss cases in Coregonus peled infested by Ergasilus sieboldi from the lake of north-west Russia. Recently, Tolba et al (2018) have reported from their study conducted on parasite infested fishes from Beni-Haroun dam that the condition index in non-infected females of Luciobarbus callensis by the ectoparasites is higher than that found in infested fish females, and that found in Foum El Khanga dam by Boucenna (2017), reporting higher condition factor in the non-parasitized specimens of Cyprinus carpio than that of the parasitized specimens. Besides, Hadji et al, (1994) have concluded that Peroderma cylindricum significantlyslows down the absolute growth in length of host fishes. Further, Ramadan (2010) has found that the copepod parasites of Mullus barbatus can result in a slowing down of its growth, however, this author and his collaborators (Ramadan et al, 2013) have noticed the presence of significant differences in Fultan’s condition index between parasitized and non-parasitized females of B.boops. Furthermore, Johnson et al, (2004) have found that many copepods species have potential importance in affecting growth, fertility, and even the monitoring of their breeding hosts. Several authors have reported that the heavily parasitized fishes by copepods suffer from anemia, affecting their growth. According to Trilles (2007), some parasites can induce death to their hosts, as evidenced in the case of Livoneca amurensis and Olencira praegustato, parasitizing respectively Leuciscus waleckii and Brevoortia tyrannus.
Conclusion
In summary, this work described herein is related to the contribution to a study of Abramis brama growthunder the effect of copepod parasites from Beni-Haroun dam (Souk-Ahras city, northeast Algeria), and thus the main results are highlighted as below:
Von Bertalanffy (1938) equation parameters of the growth study have shown different values in asymptotic length L∞, K growth coefficient, and performance index (Ø’) in non-parasitized and parasitized specimens.
The size-weight relationship of Abramis brama population was found to be negative (minor) allometry for the non-parasitized and parasitized specimens, and this is explained by the fact that size grows more rapidly than weight.
The value of “K” condition index shows that non-parasitized fishes grow better and more rapidly than parasitized fishes.
Conclusively, the following research points merit consideration in the future:
Increase the sampling effort to provide good insight into the effect of parasitism on the growth of Abramis brama population from Beni-Haroun dam.
Perform similar works investigating the growth of other cyprinid fishes, including Carassuis carassuis, Cyprinus carpio, and Luciobarbus callensis
Study the general growth and growth under the effect of copepod parasites in cyprinid fishes in other water bodies in Algeria.
References
Adlard, R.D., Lester, R.J.G. (1994). Dynamics of the interaction between the parasitic isopod, Anilocra pomacentri, and the coral reef fish, Chromis nitida. Parasitology, 109:311-324.
Al-Niaeem, K.S. (2015). Pathological studies of experimental Ichthyophonus sp. infection in blue tilapia, Oreochromis aureus (Steindachner, 1864), Iraq. Research Journal of Science and Technology, 7:234.
Avenant-Oldewage, A. (2001). Protocol for the assessment of fish health based on the Health Index. Report and manual for training of field workers to the Rand Water Board. Rand Water Board, Gauteng, South Africa.
Benmansour, B. (2001). Biodiversité et bioécologie des copépodes parasites des poissons téléostéens (Doctoral dissertation, Thèse de Doctorat, Université de Tunis El Manar).
Berrouk, H., Tolba, M., Boucenna, I., Touarfia, M., Bensouilah, M., Kaouachi, N., Boualleg, C. (2018). Copepod parasites of the gills of luciobarbus callensis (valencienne, 1842) and carassius carassius (linnaeus, 1758) (cyprinid fish) collected from beni haroun dam (Mila, Algeria). World Journal of Environmental Biosciences, 7:1-7.
Berrouk, H. (2019). Etude des crustacés ectoparasites branchiaux de l’ichtyofaune dulçaquicole du barrage Béni-Haroun-Mila (Doctoral dissertation, University of Souk Ahras).
Beyer, J.E. (1991). On length-weight relationships. Part II: Computing mean weights from length statistics. Fishbyte, 9:50-54.
Bilong Bilong, C.F. (1995). Les Monogènes parasites des poissons d’eau douce du Cameroun: biodiversité et spécificité; biologie des populations inféodées à Hemichromis fasciatus. These de Doctorat d’État, University of Yaoundé I, Yaoundé, Cameroon.
Boucenna, I., Boualleg, C., Kaouachi, N., Allalgua, A., Menasria, A., MAAZI, M.C., Mourad, B. (2015). L’infestation de la population de Cyprinus carpio (Linnaeus, 1758) par les copépodes parasites dans le barrage foum el khanga (souk-ahras, Algérie). Bulletin de la société zoologique de France, 140:163-17.
Boucenna, I. (2017). Etude des crustacés parasites de l’ichtyofaune des écosystèmes dulçaquicoles de la région de Souk-Ahras, Thèse de Doctorat, Université Chadli Benjdid, El Taref, (Algérie), p:149.
Bouhbouh, S. (2002). Etude bioécologique de deux espèces de barbeau Barbus callensis et Barbus fritschi au niveau du réservoir Allal El Fassi, Thèse de doctorat, Faculté de. Sciène. Fès, (Maroc), p:167.
Sara, B., BAROUR, C., Sameh, A., BOUALLEG, C., Mourad, B. (2016). Environmental parameters and parasitism in common carp (Cyprinus carpio Linnaeus, 1758) caught from Oubeira Lake (North-East of Algeria). Research Journal of Fisheries and Hydrobiology, 11:27-36.
Bragoni, G., Romes, T.B.,Trilles, J.P. (1983). Parasitoses a cymothoidae chez le loup (Dicentrarchus labrax,Linnaeus,1758) en elevage. 2-Ecophysiologie parasitaire dans le cas de l’etang de Diana (Haute–Corse). Annual Parasitol Hum Comp, 58:593-609.
Bush, A.O., Lafferty, K.D., Lotz, J.M., Shostak, A.W. (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited. The Journal of parasitology, pp:575-583.
Cassier, P., Brugerolle, G., Combes, C. (1988). Le parasitisme:un équilibre dynamique. Masson Paris, p:361.
Cone, D.K., Marcogliese, D.J., Watt, W.D. (1993). Metazoan parasite communities of yellow eels (Anguilla rostrata) in acidic and limed rivers of Nova Scotia. Canadian Journal of Zoology, 71:177-184.
Dagnelie, P. (1975). Théorie et méthodes statistiques. Applications agronomiques Gembloux, Presse Agronomique, pp:378-451.
Dall, W., Hill, H.J., Rothlisberg, P., Staples, D.J. (1990). The biology of the Penaeidae. Advances in Marine Biology, 27:489.
Dogiel, Y., Petrushevski, G., Polyomski, Y. (1958). Parasitology of fish. Lenin Grade Universal Press, p:184.
Esch, G.W., Fernandez, J.E. (1993). A functional biology of parasitism. Ecological and evolutionary implications. Cambridge, Great Britain. University Press, p:337.
Gayanilo, Jr.F.C., Sparre, P., Pauly, D. (2005). FAQ-JCLARM Stock Assessment Tools II(FISAT). FAQ Computerized, Information Series Fisheries, Rome, Italy, p:168.
Hadji, A. (1994). Mise en valeur et réhabilitation des Oasis, Essai d’évaluation de l’expérience Tunisienne. Montpellier, Alger, Ciheam, INFSAS.
Ibrahimi, M.M. (2012). Variation in parasite infra communities of Tilapia zillii in relation to some biotic and abiotic factors. International Journal of Zoological Research, 8:59-70.
Johnson, S.C., Treasurer, J.M., Bravo, S., Nagasawa, K., Kabata, Z. (2004). A review of the impact of parasitic copepods on marine aquaculture. Zoological Studies, 43:229-240.
Kerdoud, S. (2006). Le bassin versant de Béni Haroun, eau et pollution, Mémoire de Magister. Université de Mentouri, Constantine, p:128.
Khidr, A.A., Said, A.F., Ola, A., Abu Samak, O.A., Abu Sheref, S.F. (2012). The impacts of ecological factors on prevalence, mean intensity and seaseonal changes of the monogenean gill parasites, Microcotyloides sp, infesting the Terapon puta fish inhabiting coastal region of Mediterranean Sea at Damietta region. The Journal of Basic and Applied Zoology, 65:109-115.
Koskivaara, M., Tellervo, E.V., Prost, M. (1991). Seasonal occurrence of gyrodactylid monogeneans on the roach (Rutilus rutilus) and variation between four lakes of differing water quality in Finland. Aqua Fenn Journal of Ecology, pp:47-55.
Leveque, C., Paugy, D., Teugels, G.G. (1990). Faune de poissons d’eau douce et saumâtres de l’Afrique de l’Ouest. Faune Tropicale XXVIII, Mrac, Tervuren. Orstom, Paris, p:902.
Madanire-Moyo, G., Barson, M. (2010). Diversity of metazoan parasites of Africain catfish Clarias gariepinus (Burchell,1822) as indicators of pollution in subtropical Africain river system. Journal of Helminthes, 84:216-227.
Marcogliese, D.J., Cone, D.K. (1996). On the distribution and abundance of eels parasites in Nova Scotia:influence of pH, Journal Parasitology, 82:389-399.
Marcogliese, D.J. (2005). Parasites of the superorganism:are they indicators of Ecosystem health. International Parasitology, 35:705-716.
Margolis, L., Esch, G.W., Holmes, J.C., Kuris, A.M. et Schad, G.A. (1982). The use of ecological terms in parasitology (report of an ad hoc committee of the American Society of parasitologists). Journal of Parasitology, 68:131-133.
Oros, M., Hanzelová, V. (2009). Re-establishment of the fish parasite Fauna in the Tisa river system (Slovakia) after a catastrophic pollution event, Parasitol Research,104:1497-1506.
Pauly, D. (1979). Gill size and temperature as governing factors in fish growth: a generalization of Von Bertalanffy growth formula. Ber Inst Meereskd. Chrisatian Albrechts. University, (Kiel), 63:156.
Pauly, D. (1985). Quelques méthodes simples pour l’estimation des stocks de poissons tropicaux. FAO Doc. Tech, Pèches, p:56.
Pauly, D. (1993). Fishbyte section editorial. Naga, ICLAM Guart, p:26.
Pauly, D., Moreau, J. (1997). Méthodes pour l’évaluation des ressources halieutiques. Collection polytech de L’I.N.P.de Toulouse, Cepadues Editions, p:288.
Piasecki, W., Goodin, A.E., Eiras, J.C., Nowak, B.F. (2004). Importance of copepod in Freshwater aquaculture. Zoological Studies, 43:193-205.
Petrakis, J.M., Stergiou, K.L. (1995). Weight-length relationships for 33 species in Greek water. Fish Research, pp:465-469.
Poulin, R. (2000). Variation in the intraspecific relationship between fish length and intensity of parasitic infection. Biological and statistical causes. Journal of Fish Biology, pp:123-137.
Ramadane, Z. (2009). Identification et écologie des ectoparasites crustacés des poissons Téléostéens de la cote Est Algérien. Thèse de Doctorat, Université Badji-Mokhtar, Annaba, (Algérie), p:235.
Ramadan, Z., Amara, R., Trilles, J.P. (2010). Impact des parasites sur les performances biologiques de Mullus barbatus. L.INOC-Tishreen University, International Conference on Biodiversity of the Aquatic Environnement, p:17.
Ramadane, Z., Trilles, J.P., Mahe, K., Amara, R. (2013). Metazoan ectoparasites of two teleo fish, Boops boops, and Mullus barbatus barbatus from Algerian coast diversity, parasitological index and impact of parasitism. Cybium, pp:59-66.
Richter, H., Luckstadt, C., Focken, U., Becker, K. (2000). An improved procedure to assess fish condition on the basis of length-Weight relationships. Archive of Fishery and Marine Research, 48:255-264.
Romestand, B., Trilles, J.P. (1979). Influence des Cymothoadiens Meinertia oestroides, Meinertia parallela et Anilocra physodes (Crustaces, Isopodes, parasites de poissons) sur la croissance des poissons hôtes Boops boops et Pagellus erythrinus (Sparides). Z Parasitenk, 59:195-202.
Saila, S.B., Recksiek, C.R., Prager, M.H. (1988). Basic fishery science programs, A compunction microcomputer programs and manual of operation. Development in Aquaculture and Fisheries Science, p:231.
Singhal, R.N., Jeet, S., Davies, R.W. (1986). The relationships between changes in selected physic-chimical properties of water and occurrence of fish parasites in Haryana, India. Tropical Ecology, 27:1-9.
Stavrescu-Bedivan, M.M., Popa, O.P. (2014). Infestation of Lernaea cyprinacea (Copepoda :Lernaeidae) in tow invasive fish in Romania, Lepomis gibbosus and Pseudorasbora parva. Knowledge Management Aquatic Ecosystems, p:414.
Sparre, P., Venema, S.C. (1996). Introduction à l’évaluation des stocks de poissons tropicaux, Première partie:Manuel FAQ, Document technique sur les pèches N306/2. Revue 1Rome, p:106.
Tolba, M., Kaouachi, N., Boualleg, C., Mouaissia, W., Allalga, A., Berrouk, H., Boulahbal, S. (2018). Impact of parasitic helminths on the growth of luciobarbus callensis populating béni haroun dam (East of Algeria). World Journal of Environmental Biosciences, 7:92-99.
Trilles, J.P. (2007). Olencira praegustator (Crustacea,Isopoda,Cymothoidae) parasitic on Brevoortia species (Pisces,Clupeidae) from the Southeastern coasts of North America: review and redescription. Marine Biology Research, 3:296-311.
Von Bertallanffy, L. (1938). A quantitative theory of organic growth (Inquiries on growth laws II). Human Biology, 10:181-213.
Wahle, A.R., Fogarty, M. (2006). Growth and development: understanding and modeling variability in lobsters: biology, management, aquaculture and fisheries. Oxford, Blackwell, pp:1-44.
Author Info
B. Houda1,2*, S. Fatiha1,2, K. Nouha1,2 and B. Chahinaiz22Faculty of Natural and Life Sciences, Mohamed El-Cherif Messaadia University, Souk-Ahras, Algeria
Citation: Houda, B., Fatiha, S., Nouha, K., Chahinaiz, B. (2021). Effect of parasitic copepods on the growth of Abramis brama fish from Beni-Haroun dam of Mila city (Northeast Algeria). Ukrainian Journal of Ecology 11 (8), 79-88.
Received: 08-Sep-2021 Accepted: 11-Oct-2021 Published: 23-Oct-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
