Research - (2021) Volume 0, Issue 0
Eurydema bugs: review of distribution, ecology, harmfulness, and control
S. Stankevych1*, I. Zabrodina1, D. Yushchuk1, M. Dolya3, H. Balan2, R. Yakovlev3, H. Kosylovych4, Yu. Holiachuk4, N. Zakharchuk5, T. Galagan6, L. Nemerytska7, I. Zhuravska7, O. Romanov1, T. Romanova1, O. Bragin1, O. Hudym1 and I. Hordiienko1Abstract
In the course of the literature critical analysis, the authors paid special attention to the morphological, biological, and ecological features of the cruciferous bugs, both in Ukraine and abroad; the authors came to the conclusion that despite the considerable number of literary sources devoted to the cruciferous bugs, theirs is still a number of its biological and ecological features which are in close connection with the protection measures for controlling it and these measures have not yet been completely clarified. The data obtained by entomologists from different countries regarding the harmfulness of thecruciferous bugs and its economic importance are quite controversial and also need experimental confirmation.
Keywords
Cruciferous bugs, Morphology, Biology, Ecology, Harmfulness, Economic threshold of harmfulness, Integrated protection.
Introduction
The complex of cruciferous bugs includes species such as painted or harlequin (cabbage) bug (Eurydema ventralis Kol), pentatomid rape bug (E. oleraracea L.) and mustard bug (E. ornata L.). They belong to a Hemiptera line, the Shield bug family (Pentatomidae), and the Cruciferous bug genus Cruciferous (Eurydema). Cruciferous bugs are a common species and are spread throughout the Palaearctic. They are widespread throughout the whole territory of Ukraine (Puchkov, 1961; Yevtushenko et al., 2016). Adult bugs and larvae cause damage to crops; they pierce the skin of the leaves or the floriferous shoots with the proboscis and sucking out the juice. Light spots appear at the puncture points, the tissue dies, falls out, and irregular forms of holes form. When seeds are damaged, the flowers and ovary fall off and the quality of the seeds deteriorates. The economic threshold of harmfulness is 2 to 3 bugs per plant (Puchkov, 1961; Yevtushenko et al., 2009; Stankevych, Vilna, 2012; Vilna, 2013; Vilna, Stankevych, 2013; Stankevych, Kava, 2013; Yevtushenko, Vilna, 2014a; Vilna et al., 2015; Stankevych, 2015; Yevtushenko et al., 2016).
Materials and Methods
The authors analyzed 160 literary and electronic sources from the late 19th to the 20th century. During the analysis, special attention was paid to the morphological, biological, and ecological characteristics of cruciferous bugs in Ukraine and abroad. Data on the harmfulness of flea beetles and their economic importance are especially analyzed. In the course of the analysis, special attention was paid to the methods and ways of controlling the cruciferous bugs in Ukraine and abroad. The protective measures were considered in such directions as agrotechnical, physic, mechanical, chemical, biological, biotechnical, selective, and genetic. Each of them is noteworthy and has both a number of disadvantages and indisputable advantages in comparison with other methods.
Results and Discussion
The taxonomic status of Eurydema bugs (Eurydema spp.): Class Insects-Insecta; Order Hemipteranss or Bugs-Hemiptera; Family Pentatomids-Pentatomidae; Genus Eurydema.
Habitats
The vast majority of representatives of the superfamily Pentatomoida live and reproduce their kind mainly on herbaceous plants, less often on shrubs and trees. However, almost all shield bugs use the soil as overwintering housings, lying in its upper layers or among the plant detritus that covers the soil (Agapova, 1953; Puchkov, 1961).
Shield bugs, like other groups of terrestrial hemipterans, are mostly xerophilic, inhabiting well-warmed biotopes. Soil-related species prefer light soils (sandy, loamy), while dendrobionts are concentrated in forest edges, single trees, and shrubs; in forests, dendrobionts inhabit the tops of crowns, where there is more light and heat. Only a small number of shield bugs propagate themselves in swampy or humid, heavily shaded, and fairly cool places (Bogachev, 1941).
Shield bugs, like other insects, can change (to some extent) the choice of habitats in various landscape-geographical zones (Bej-Bienko, 1930) due to certain constant requirements of the species to a hydrothermal mode. These requirements are intrinsic to the species in any place of the range (Arnoldi, 1952), in particular, with as the temperature increases, insects need higher relative humidity, and when it decreases, they move to drier places. SI Medvedev proposed a clear system of ecological groups of insects based on their adaptation to the typical conditions of landscape-geographical zones and subzones of Ukraine (Medvedev, 1954). This system outlines the peculiarities of the distribution of bugs, including shield bugs, in Ukraine.
Overwinter housings
In the vast majority of shield bugs, adult insects (imagoes) overwinter. Depending on the species and physiological state of the shield bugs, the time of their departure for the winter can differ. Some shield bugs move to overwintering housings as early as mid-June, although most shield bugs can only be found there (in the forest-steppe) in September. Shield bugs often overwinter in or near reproduction sites, but some species make more or less distant migrations from overwintering housings to reproduction sites, and vice versa. All shield bugs migrate to more or less dry places for the winter (Bodrenkov, 1927; Vilna, 2013c; Vilna, 2014a; Yevtushenko and Vilna, 2014a; Yevtushenko et al., 2015). When inspecting the areas that shield bugs use for overwintering, in late autumn, winter, or early spring, one can sometimes find rather large numbers of shield bugs,, leaf-footed bugs and lygaeid bugs among the plant remains under single trees and shrubs. They always station themselves in the forest litter in siglles (Vodolagin, 1936). Some species of shield bugs choose to overwinter in places protected by shrubs, trees, or remnants of tall grasses, others migrate to places with low herbaceous vegetation, but they all overwinter in the forest litter, topsoil, under rocks, and other shelters. Some shield bugs tend to overwinter in tree hollows, under exfoliated bark, in bird nests, under the roofs of various buildings, in cones of coniferous trees, or even on branches among dense conifer needles (Stark, 1928; Vilna, 2014a; Yevtushenko and Vilna, 2014a; Yevtushenko et al., 2015).
Spring awakening of shield bugs
In spring, some species of shield bugs awaken in winter housings immediately after snow melts away and the forest litter, in which the shield bugs lie, warms up to 6-10°C. Most shield bugs, which overwintered, become active only when the forest litter (or soil) temperature rises to 10-12°C (Arnoldi, 1947; Masse, 1954). Depending on the microclimate of overwintering housings, the emergence of shield bugs more or less continues; in addition, the period of spring awakening may be influenced in some way by the physiological state of insects (Giterman, 1931). However, some species of shield bugs usually leave their overwintering housings more en masse and evenly than they depart for overwintering. After leaving their winter housings, shield bugs remain near these places for some time. They can be found in various plants and even feed on their sap, but later, before laying eggs, shield bugs move to their main food plants, where they lay eggs and larvae develop later (Velichkovskij, 1913; Yevtushenko and Stankevich, 2012; Stankevich and Vilna, 2013; Vilna and Bondar, 2013; Vilna, 2014b; Yevtushenko et al., 2015).
Mature and mating of the gonad
The interval between feeding recommencement and egg laying may be different. The gonads of shield bugs can develop quite quickly; sometimes their development begins before the feeding recommencement. Some species do not require a certain quality of food for the development of gonads or quickly complete development when such food is available (Kirichenko, 1925). At a high temperature (18-20°C) after overwintering, shield bugs start mating almost immediately after feeding recommencement. The shield bugs mate several times. The mating act lasts from 0.5-2 to 10 hours or longer (Puchkov, 1956a).
Egg laying
In the forest-steppe of Ukraine, shield bugs begin to lay eggs en masse from the end of May to the end of June, especially intensively in the first half of June; in the South, these deadlines are one to two weeks earlier. Although shield bugs that overwintered begin to die mainly in the second half of June-early July and their numbers decrease, some females of many species survive and even lay eggs until August (Shrejner, 1915; Yevtushenko et al., 2012; Vilna and Stankevich, 2013). The embryonic development of shield bugs, depending on environmental conditions, especially temperature, lasts 3-20 days, although it usually ends within 5-12 days (Korinek, 1939; Fedorenko, 2014).
Larva hatching and development
The process of hatching larvae from eggs is similar in all shield bugs. The larva, which has completed its development, breaks the chorion from the inside with its egg opener gibbosity. The chorion gradually cracks along the edge of the groove that runs along the lid edge (Pentatomidae). The larva then gradually squeezes through the hole and exuviates the embryonic skin together with the egg opener at the hatching moment. The whole hatching process of the larvae lasts about 8-10 minutes (Puchkov, 1961).
If the eggs were laid in a cluster, the larvae remain in a dense clump on empty shells for some time. In special studies, it was shown that in many species of shield bugs there were specific fungi and symbiotic bacteria on the surface of the chorion, which get there with the female secretions that bind the eggs. The larva absorbs these secretions, and the symbionts, entering the larval intestine, reproduce themselves in it and facilitate the assimilation of food by the body (Shapiro, 1951b). The first-instar larvae of other insects are also known for the need for swallowing symbionts (Carayon, 1949). The mechanism of transmission of insect symbionts from parents to offspring was revised by J. Carayon (Carayon, 1952); in shield bugs, symbionts get on the egg surface with intestinal secretions, or larvae directly absorb droplets of secretion evacuating from the female's anus and containing symbionts (Schorr, 1954). Symbionts are applied to eggs with a special organ located in the female's genitals. This organ consists of a large number of thin tubes filled with symbionts (Rosenkranz, 1939).
The desire for thronging, which is typical for larvae of most species of shield bugs laying eggs in clusters, is inherent only to the first instar. Larvae of some species crawl away a few hours after hatching from eggs, while others leave the clutch only a few days before ecdysis to the second instar. The duration of the larval development depends on temperature and humidity in places where larvae live, food availability, and quality. The development of shield bug larvae lasts on average 25-35 to 40-55 days (in most species) (Fedorenko, 2014). The vast majority of shield bugs have one generation per year, and only a small number of species develop two generations in Ukraine (Pachosskij, 1889, Vilna, 2013a).
Color change in imagoes and larvae
The autumn pigmentation of many shield bug species differs from that in spring, which is especially noticeable in species that are green in spring (Reuter, 1907; Kirichenko, 1908; Masse, 1954; Leston, 1955). The difference between autumn and spring pigmentations is not a simple reaction to temperature and other meteorological factors. It is known that parasite-infested shield bugs often have off-season coloration. Less abruptly and not in all specimens, the seasonal change in color is observed in other bugs, not green shield bugs. Carpocoris sp.,, Dolicoris sp. and other hemipterans are darker in autumn than in spring; species of the genus Eurydema are prone to significant seasonal and off-season variability (Rusanova, 1926; Hlebnikova, 1927; Mihajlov, 1949; Teyrovsky, 1949). In these species, there is a more or less definite relationship between the pigmentation of the outer investment and the status of the gonads. However, for many species of the superfamily, especially in the family Pentatomidae (true shield bugs), the third/fourth-instar larvae are usually dark or light-colored larvae; this feature is manifested later in imagoes. The dark color of the cuticle is the result of a more intense deposition of melanin. This, as in Ch. Dupuis (Dupuis, 1949) demonstrated that slowing down the metabolism is attributed to exposure to low temperatures as well as to other causes that weaken the activity of insects, in particular to parasite invasions. Dark-colored larvae are seen more frequently in fall, at the end of population development. These larvae are usually smaller than normal ones. Furthermore, the number of dark larvae increases in years with cold and rainy growth periods. Dark larvae are more common in shaded areas, in northern and alpine locations, while in the south larvae of many shield bug species are lighter, and some parts of their bodies are rather bright (often red) (Kirichenko, 1908, Puchkov, 1956b).
The pigmentation variability of some parts of the body, which is intrinsic to some genera (especially the pattern variability on the pronotum, clypeus, corium), served as a basis for the description of a large number of forms or variations in many species, and each of them was given a Latin name. Especially many forms (more than 30) are described for the rape bug (Eurydema oleracea); a little fewer for Eurydema ornata, and the numbers of newly described forms of these species are growing. These descriptions are mostly based on slight changes in the pattern or other unstable features inherent in the species; however, they have neither scientific nor practical significance. The described forms and variations only complicate the identification of ‘real’ forms (sometimes even subspecies), which differ from the typical form by several morphological and ecological features (Dupuis, 1949; Puchkov, 1956a).
Trophic groups and food relations
Among shield bugs, there are representatives of almost all five major trophic groups of herbivorous hemipterans (Puchkov, 1956b). These trophic groups of herbivorous hemipterans evolved as a result of a rather long process of adaptation of shield bugs to feeding on certain parts of plants, which made significant physiological changes in the structure of many species. Hemipteran larvae are especially used to feeding on certain parts of plants (Vilna, 2013c, Vilna, 2013d). In numerous experiments, it was proven that the mortality of larvae that ate unsavory parts of edible plants always increased dramatically. However, there are no clearly defined boundaries between trophic groups within hemipterans, and many species, depending on the developmental stage and the impact of environmental conditions, may become closer to one group or another. For these species (they are especially numerous among shield bugs), it is vital to replace some parts of plants with others during their development. However, the replacement of some parts of a particular plant species with others is only one aspect in changes of the food relations of bugs. In any trophic group of hemipterans, oligophages and polyphages are represented, which expand or narrow their food relations during the development of larval and adult stages. The food relations of the hemipteran larvae are much narrower than those of the imagoes. Early instar larvae have the fewest number of plants they can eat. At subsequent stages, it may not change, especially in the fifth-instar larvae. The species diversity of food relations in young imagoes is usually only slightly greater than in the fifth-instar larvae (Kirichenko and Talickij, 1933; Puchkov, 1956b).
During the autumn feeding, older larvae especially young imagoes of shield bugs form fat bodies; the organism gets water by sucking succulent parts of plants, dew or rain droplets, etc. Not all plants change their seed composition under the influence of the salivary enzymes of shield bugs; not all seeds become suitable for absorption and assimilation. The set and activity of salivary enzymes in different species of hemipterans may be different. Furthermore, without replenishing the water required at least for saliva production, shield bugs can not feed on dry seeds for a long time. Thus, shield bugs feed on plants whose seeds they digest well and in places where water is available to them (Pazhitnova, 1952). The food relations of many herbivorous bug species are also expanded by including animal-driven food in their diet. Many typical phytophagous bugs in the larval and adult stages feed on animal feces and suck eggs of different insects (sometimes of their own kind). However, such nutrition is not mandatory for their normal development (Puchkov, 1956b). The food relations of shield bugs, like other hemipterans, are still poorly studied, and the vast majority of publications are only limited by observations of imagoes. Published data on some bug species clearly indicate an insignificant variability in trophic relationships (Arnoldi, 1957).
Feeding process and damage to plants
Specialization in feeding certain parts of edible plants has led to considerable physiological changes in many species. These changes facilitate the extraction of nutrients from certain parts of plants and the assimilation of food. The physiological changes are inevitably manifested in the nature of plant injuries induced by hemipterans. The signs of these injuries are typical for all species in a particular trophic group. This, on the one hand, allows one to know in advance features of damage that can be caused by certain bugs species to different wild and cultivated plants and the impact of this damage on the plant development, and, on the other hand, facilitates the detection of food plants of bugs in nature (Bondarovich, 1926; Stankevich and Vilna, 2012d; Yevtushenko and Stankevich, 2012; Vilna, 2014b; Yevtushenko et al., 2014a; Vilna et al., 2015; Vilna, 2015).
The feeding process in shield bugs can be described as follows: the insect, affixing its proboscis to a certain place on the plant, inserts sharp stilettos into the tissue. However, stilettos can bend, turn from side to side, move back and forth, incline at various angles, penetrate between or into cells, but no traces other than torn cells are left in the way of their movement (unlike aphids and cicadas). Feeding, shield bugs not only suck the sap of plants, but also inject their saliva into the tissues. The amount of injected saliva is very small compared to the amount of sucked sap (Flemion et al., 1952). Damage caused by bugs in plants consists of the mechanical destruction of plant tissues by stilettos and the toxic effects of saliva injected during feeding into the tissues. Due to bug-induced damage, plants suffer from more or less severe pathological abnormalities (Yakovlev, 1874). Recently, indirect and direct experiments have shown that saliva not only causes tissue cell death near the bug's feeding site and gradually expands the necrotic area, but it can also spread from the injection site throughout the plant, weakening the plant and delaying its development. Among shield bugs, there are few species in trophic group 3 (Cabbage bug Eurydema ventralis), which are close to group 4 in terms of damage nature. The species of trophic group 4 feed mainly on fleshy tissues, vascular bundles of phloem and fruits. They suck out buds, leaf veins, young parts of stems, and generative parts of plants, where the influx of nutrients is most intense (Bondarovich, 1926).
The saliva of group 4 bugs exerts an irritating effect, enhancing the inflow of nutrients to the puncture site. The negative effects of saliva on the plant are not limited to cell destruction or general plant poisoning (Yakovlev, 1874).
The importance of bug saliva is also attributed to the fact that it makes the substances contained in one or another part of the plant suitable for suction and assimilation. Thinning of the contents of ripe plant seeds by bug saliva was first described in 1914 and later by Kretovich et al. (1943). This role of saliva is very important for the life of many shield bugs and for some species of other families, which are able to feed on the contents of ripe and ripening seeds during certain periods of development, acquiring the characteristics of trophic group 5. Saliva from all bugs in this group contains special enzymes such as amylase, which allow the use of solid starch and other substances for nutrition. Being injected into fruits/seeds, the enzymes do not spread outside the bug's feeding zone (Yakovlev, 1874). The puncture site made by stilettos in plant tissue is initially almost invisible even under binoculars, but when the bug removes its stilettos from the tissue, a drop of sap exudes on the plant (though not always). The admixture of bug saliva in this drop sometimes necrotizes the underlying tissue cells, and then a spot appears around the puncture site. The color of the spot depends on the chemical composition of the sap of the damaged plant. It can be white, brown, or even black. Sometimes, there is no spot under the drop and only a trace of its dried content remains (Vilna, 2013d; Vilna and Stankevich, 2013; Vilna, 2014b; Yevtushenko and Vilna, 2014a; Yevtushenko et al., 2015). The puncture site is significantly complicated when various pathogens enter the tissue (Yakovlev, 1874).
As a result of bug feeding, the normal condition and development of plants is disturbed, which is associated with different malformations. When the growth points of young plants are damaged, they branch. Damage to the generative organs of plants causes the death of flower buds, leaf buds, flowers or whole inflorescences and young fruits (Teslina and Stankevich, 2010, Stankevich and Vilna, 2012a, Stankevich and Kava, 2013, Stankevich and Vilna, 2014ab, Stankevich, 2015). Seeds damaged in the early stages of development usually die by drying in a thin plate. If damage is inflicted at the end of the milky ripeness phase or later, the seeds do not die, although they are smaller than normal ones, germinate more slowly and shrink. The damaged seeds look very similar to healthy ones and differ only due to the presence of a small dot, the puncture site, and the dilution of the fruit contents under the influence of salivary enzymes. Saliva residues reduce the appeal to consumers of damaged seeds, and the negative impact of saliva residues of different species of bugs can be different. If the corcules of the ripe seeds are damaged, their germinability drops to zero (Stankevich and Vilna, 2012a; Stankevich, 2013; Stankevich and Vilna, 2013; Vilna, 2014b; Vilna and Stankevic, 2014; Vilna et al., 2014; Vilna, 2015; Yevtushenko et al., 2015).
Parasitoids
Shield bugs have many enemies, parasites, and are prone to different pathogens, the species composition of which has been studied very little, as few harmful species are known. As to vertebrates, various insectivorous mammals, especially hedgehogs, some rodents (mice), and even pigs, are willing to eat shield bugs, but particularly large numbers of bugs are consumed by various wild birds. The relatively large size and hard coverings of the body protect imagoes from attacks of predatory invertebrates, and they are attacked only by bigger bugs, but larvae of shield bugs are often pursued by even small predators. At the adult and larval stages, shield bugs are infested by larvae of tachina flies (Belanovskij, 1951; Belanovskij, 1953), which sometimes significantly reduce bug populations. However, shield bugs are the most vulnerable in the egg stage; their eggs are eaten not only by numerous predators but also by herbivorous invertebrates, in addition to specialized parasites, egg eaters (Fasulati, 1954).
Superfamily Pentatomoidea.
Imago
The body is short and oval or oval, sometimes almost round. The head has conspicuous, more or less flattened parafacialia, which cover the top of the antenna protuberance. The antennae are five-segmented (in all species in Ukraine). The shield is large, reaching the diaphragm and at least to the middle of the abdomen, sometimes to its top. The legs are two-segmented (Shrejner, 1915; Fedorenko, 2014).
Larva
The body is short and oval, rarely round. The antennae are four-segmented. The legs are two-segmented. The abdomen has three pairs of orifices of the smell glands. Evaporation areas are usually darker than the background of the abdomen. The spiracle disposition is similar to that in imagoes.
Genus Eurydema
Imago
The body is elongated oval, bright, black, blue, or dark green, metalescent, with a sharply outlined red, yellow, or white pattern, dotted with scattered colorless dots, completely naked (Saharov, 1934; Fedorenko, 2014).
The head is moderately inclined, trapezoidally rounded in front, and quite strongly bent on the sides in front of the eyes. The parafacialia on the outer edge are bordered by a high elevated thick rounded rib, longer than the clypeus, and, in contact with the inner edges, completely cover its top. The eyes are large, strongly convex, but not stalked. The antennae are black, covered with short hairs (Puchkov, 1961; Fedorenko, 2014). The anterior and lateral angles of the pronotum are bordered by a continuous (without gaps) high-rounded rib, thicker in the anterior pronotum, and slightly narrowed towards its lateral angles. The lateral angles of the pronotum are rounded, do not protrude, or barely protrude beyond the outer edge of the elytrons. The shield is markedly narrowed towards the top. The corium is longer than the shield, about 2/3 of the length of the outer edge is bordered by a raised, rounded rib, which gradually gets narrowed from the base (Shrejner, 1915).
Larva
YeN Polivanova described the larva as follows (Polivanova, 1956): the body is egg-shaped (1st-3rd instar) or oval (3rd-4th instar), shiny, naked, dotted with small colorless black dots scattered and visible only in older larvae. The head and thorax on top are completely black or brown (1st-3rd instar), or black with a more or less obvious light pattern of the same color as the abdomen (4th-5th instar). The head in front is narrowed trapezoidally. The parafacialia on the outer edge are bordered by a rather high and thick, roller-like rib (2nd-5th instar). The clypeus is longer than (1st instar), as long as (II) or shorter than (3rd-5th instar) the parafacialia; in the fourth and fifth instar larvae, it is covered by the parafacialia in front. The eyes are large, almost round, and strongly convex, dark brown or black (1st-5th instar). The antennae are dark brown (1st-3rd instar) or black (1st-5th instar), covered with short hairs (Puchkov, 1956a). The anterior edge of the pronotum is back and the lateral edges of the thorax are bordered by a rounded roller-like rib (2nd-5th instar). The legs are covered with light hairs (Polivanova, 1956). The abdomen has black, evaporating areas, which are sharply defined spots in all instars, at the base of tergites VII and VIII, paratergitic, parasternitic, and sternitic spots (the latter are sometimes fuzzy). The bottom of the abdomen is the same color as the top, with large sternitic spots, which gradually decrease after the spot on sternite VI (Puchkov, 1956a).
Economic significance
According to data of Suhorukov (1947), Shapiro (1951a), Suhorukov (1953), and Volovik (1953), among the species of the genus Eurydema, there are species doing great harm in some areas of Ukraine. Eurydema bugs are closely associated with many wild (Vilna and Bondar, 2013; Vilna et al., 2015) and cultivated Brassicaceae plants, in the vegetative and generative parts of which larvae and imagoes feed (Agapova, 1953; Korolkov, 1929). Less often, adult bugs, and sometimes older larvae, feed on plants of other families, even on Gramineae plants. The domestic names of Eurydema bugs are very confusing and often different researchers give the same name to different species. This confusion is exacerbated by using special, completely unnecessary names for color forms. The best names were proposed by ID Shapiro (Shapiro, 1951a). In Ukraine, Eurydema bugs include the cabbage bug Eurydema ventralis Kol., the rape bugs E. oleracea L., E. ornata L.
Cabbage bug (Eurydema ventralis Kol.)
The body of the imago is slightly flattened at the top; its sides are straightened to the miidle, almost parallel. The head is black, often with a light spot in front of the eyes and a notch on the top. The parafacialia and clypeus are dotted, without wrinkles or with slight wrinkles. The rib that borders the parafacialia is equally elevated on the sides and at the top of the head, completely light or in the anterior half. The second segment of the antennae is a third longer than the third (Kirichenko, 1915).
The pronotum is red with six black spots (two of them are anterior and four posterior) (Fig. 1); the anterior ones are separated from the posterior ones by a transverse elevation. Rarely do anterior spots merge with posterior ones, and then two spots are formed on the pronotum (like in E. spectabilis) (Bondarovich, 1926; Rusanova, 1926).

Fig 1: Cabbage bugs.
The shield with a large black spot at the base and two oblong spots on the sides, in front of the shield top. The lateral edges of the shield from the base to the pre-top spots are completely light or light at least near the middle. There is a well-marked rib running along the middle at the top of the shield. The clavus, the base of the part of the mesocorium and the mesocorium part adjacent to the clavus, the large spot in the middle of the mesocorium, the rounded spot in front of the outer apical angle of the corium and the oblong spot in the middle of the exocorium are black; the exocorium in front and behind the middle black spot is usually in the same color. The diaphragm is black with a white border. The abdominal rim is red, with large black spots on the top and bottom of the anterior part of the segments. The upper surface of the abdomen is red; the lower surface has a large black solid spot, which occupies the entire middle of the abdomen or divides into individual spots. The spiracles are often located on oval black spots (Puchkov, 1961). The anterior genital plates on the upper edge are straightened or curved, without a notch and with rounded apical inner angles. The parameres in the apical part are slightly less puffed up than in E. spectabilis, and the lobe in the middle of the apical edge of the pygidium is almost the same as in this species. The length is 9-10 mm (Bramson, 1894). The larvae of E. ventralis, except for imaginal differences, which are seen slightly in the last stages only, do not differ from the larvae of E. festiva (Grinev, 1925).
Range. E. ventralis can be found throughout Ukraine, in Moldova, in some parts of the Caucasus, Central Asia, and Western Siberia. The northern limit of the cabbage bug range passes through Grodno-Bryansk-Kazan-Chelyabinsk-Omsk-Tomsk, mainly coinciding with the southern boundary of coniferous forests, and does not go higher than 57-58° North. Latitude (Shapiro, 1951a). The cabbage bug is also common in some countries in the Middle East, North Africa, and Western Europe (to Poland). In Ukraine, it can be found sporadically and, although it sometimes propagates in vegetable gardens in large numbers, it does not inflict such severe damage as in the Volga region or in the North Caucasus, where it is most numerous (Kirichenko, 1916).
Biology and ecology. The cabbage bug overwinters under fallen leaves and leaves from plants in gardens, parks, forest edges and abandoned fields overgrown with tall herbaceous plants. It is more common near cabbage plantations (Vilna, 2013a; 2013c; Vilna, 2013d; Vilna and Stankevich, 2013; Vilna, 2014a; Fedorenko, 2014). In the South (Krymska Oblast, Astrakhan Region, Krasnodar Territory), bugs usually awaken and leave their overwintering shelters in mid-April; in the northern part of the range (Kharkivska Oblast, Voronezh and Omsk Regions)-in late April-early May; in the Zakarpatska Oblast, the first bugs were found in early April (Roshko, 1953).
After leaving their shelters, bugs stay for some time on wild crucifers, settling in different habitats, but, from early spring, Eurydema ventralis is more common than E. oleracea or E. ornata in gardens and, after cabbage seedlings and seed plants are planted, the cabbage bug resets on them (Shegolev et al., 1937; Teslina and Stankevich, 2010; Stankevich and Vilna, 2012c; Stankevich and Vilna, 2012d; Vilna and Bondar, 2013; Vilna and Stankevich, 2013; Vilna et al., 2015). In Crimea, the Lower Volga region and the Fore-Caucasus, females begin to lay eggs in late April-early May, while in the forest-steppe zone-during the second or third 10 days of May. Females that overwintered lay eggs for about 1 to 1.5 months (Kirichenko, 1928). Bugs lay eggs on various plants (sometimes on such plants that are not food for larvae), placing them on the upper and lower surfaces of leaves, on branches, stems, buds, flowers, and rarely on plant fragments. In a clutch, there are usually 12 eggs placed in two regular rows (Fig. 2) (Kalyanov, 1911). In the forest steppe of Ukraine, the development of eggs lasts 5-13 days and that of larvae-35-45 days. At an average daily air temperature of approximately 20, 24°C, the development of the first instar larvae lasts 3-4 days; of the second instar <5-6 days; of the third instar <6-9 days; of the fourth instar <78 days; and of the fifth instar <approximately 8-10 days (Shapiro, 1951a; Vilna, 2013c; Vilna, 2013d; Fedorenko, 2014). The first-to third-instar larvae have an egg-shaped body (Fig. 3).

Fig 2: Eurydema bug hatching larvae in cabbage seed plants at the Doslidne Pole of the Training, Research and Production Center (Experimental Field) of VV Dokuchaev KhNAU (07/06/2009).

Fig 3: Ecdysis of the first-instar larva (14/06/2013).

Fig 4: White cabbage seed plants damaged by Eurydema bugs at the Doslidne pole of the Training, Research and Production Center (Experimental Field) of VV Dokuchaev KhNAU (19/05/2015).
In natural biotopes, the cabbage bug develops in Descurainia Sophia(flixweed), Sisymbrium volgense, Sisymbrium Loeselii, Erysimum rеpаndum (spreading wallflower), Nasturtium austriacum (Austrian yellowcress), Thlaspi arvense (field pennycress), Crambe tatarica, Capsella bursa pastoris (shepherd's purse), Sinapis arvensis (charlock mustard), Lepidium draba(hoary cress), Brassica campestris, as well as on plats of other families-in Tropaeolum majus(garden nasturtium) and Capparis spinosa (Flinders rose). It developed en masse on C. spinosa in Crimea (Karadah) (Vilna and Bondar, 2013; Vilna et al., 2015).
ID Shapiro (Shapiro, 1951b) noted a well-defined adaptation of the cabbage bug to feeding on the vegetative parts of plants (cabbage, winter cress, mustard, etc.). ID Shapiro's experiments showed that when E. ventralis larvae fed on leaves of any of the above three plants only, their mortality was two to three times lower, and the development was shorter than in E. oleracea and E. ornata larvae, which fed under the same conditions. The feeding of larvae of all three species of bugs only on the generative parts (pods) of the same plants was more successful, but E. ventralis larvae developed much more slowly than E. oleracea and E. ornata larvae. The first generation young imagoes fly en masse in the second half of June (Crimea, Fore-Caucasus), in early July (forest-steppe). Winged, many bugs, which developed on wild crucifers, fly to cabbage, mustard, and other cruciferous crops. During this period, pests inhabit cabbage plantations in bottomlands of large rivers. Sometimes bugs swarm in such large numbers that they completely devour all cabbage plants within 3-4 hours (Kalyanov, 1911; Porchinskij, 1909; Fedorenko, 2014). Nine to 11 days after winging, the first-generation females begin to lay eggs.
The fertility of overwintered females usually does not exceed 48-88 eggs, while first generation females can lay 240-252 eggs (Saharov, 1934; Korinek, 1940), but on average they lay about 80-120 eggs. The fertility of different generations of the cabbage bug tends to vary greatly depending on environmental conditions, but they are always more favorable during the development of the first generation of the pest. The fertility of the second-generation females provided with food on cabbage plantations is not much inferior to the fertility of the first-generation bugs and averages 60-80 eggs (Shogolev and Strukova, 1932). In the northern part of the range (for example, in the Omsk region), the cabbage bug has one generation per year, while in the forest-steppe and in some places of the steppe (Voronezh region, Kharkivska Oblast and further south) it has two generations, and the second generation develops in July-August. In the South (Krymska Oblast, Rostov region, Krasnodar Territory, Transcaucasia), the third generation of the pest develops in August-October (mainly in cabbage plantations) (Porchinskij, 1909).
Generations of the cabbage bug overlap; hence, in the South, egg laying occurs from May to autumn. In addition, eggs are laid and larvae also hatch continuously in the autumn. In the vicinity of Rostov-on-Don, quite viable egg clutches were found even on November 13, although there were frosts and the temperature dropped to-4 ° C during the first 10 days of November (Saharov, 1934). With cold weather set in, some larvae of the last generation, not reaching the adult stage, die, and young imagoes often go to overwintering without sufficient fat stores. This phenomenon was observed for the third generation of the pest in the Rostov region and partially for the second generation further north (Voronezh region) (Korinek, 1940). In the first half of September (forest-steppe), in late September and in October (Fore-Caucasus), the last generation imagoes migrate to overwintering housings. Males and females have underdeveloped gonads that develop in the spring of next year (Agapova, 1953).
Economic significance
The harmful activity of the cabbage bug in the Russian Empire was first noted in 1861, when the bug completely devoured the planted cabbage in the Khvalynsk Uyezd of the Saratov Governorate. In subsequent years, the cabbage bug often damaged cabbage in the Stavropol and Krasnodar Territories, in the Stalingrad, Saratov, and Rostov Regions, and in the Astrakhan Region it was a ‘real scourge’ of cabbage from 1911 to 1924. It slightly less often inflicted damage in the Ulyanovsk Region, Kharkivska Oblast, and on the Black Sea coast of the Caucasus. It also caused significant damage in the Zakarpatska Oblast and Omsk Region. The cabbage bug also damaged mustard, turnip, rapeseed, seed plants of radish, and colewort, but it usually caused little damage to these crops, ranging on them in small numbers (Keppen, 1883; Saharov, 1930; Saharov, 1934; Shegolev, Znamenskij and Bej-Bienko, 1937). At the Doslidne Pole (Experimental Field) of the Training, Research, and Production Center of VV Dokuchaev KhNAU, the cabbage bug considerably damaged the seed plants of white cabbage.
Parasites. Oencyrtus telenomicida Vas., Trissolcus simoni Mayr., Microphanurus vassilievi Mayr. and Aphanurus eurydemae Vas. They are mentioned as parasites of E. ventralis eggs. Clytomyia continua Pz. And Phasia crassipennis F. parasitizes in imagoes and larvae of Euridema bugs. Among the parasites of the cabbage bug, T. simoni is of great importance, as it can give up to 10 generations during the summer. T. simoni is proposed for biological pest control in the Krasnodar Territory (Suhorukov, 1953). NL Saharov (Saharov, 1934) proposed using Aphanurus eurydemae Vas. to control Euridema bugs.
Eurydema ornata L.
The imago’s head almost always has a large light spot in front of the eyes. The rib that borders the outer edges of the parafacialia is flattened at the top of the head, not elevated above the parafacial level. The second segment of the antennae is 1/2-1/3 longer than the third (Bramson, 1894). The pronotum is yellow with six black spots (Fig. 5). The shield in its upper half has no longitudinal rib, or it is very indistinct. The exocorium behind the middle black spot is almost always a different color, lighter than in the front (grayish, bluish, yellowish). The abdominal rim is light (red, yellow-Kuzmina, 1937). The anterior genital plates have a straight apical edge and always have a small obtuse notch on it; the inner apical angles of the plates are rectangular. The apical angles of the blade located in the middle part of the apical edge of the pygidium are elongated at the tip, and the apical part of the parameres is very thin. The length is 7.0 to 8.5 mm (Kuznecov, 1932). The head of the larva is one-colored, brown (first instar),, black (second instar) or light (white, yellowish or ocher) with black outer edges of the parafacialia at their apex, black clypeus, and the entire back of the head to the level of the front edges of the eyes (Roshko, 1955). The thorax is completely brown (first instar), black with light lateral edges of the pronotum (second instar) or its front edge lighter too and a large spot in the middle (third instar), sometimes the entire pronotum is light, except for two large black spots on the sides, and the mesonotum is black with light spots or stripes on the sides of the shield and the middle part of the lateral edge of the elytrons (fourth and fifth instars). The legs are dark brown (first and second instars), yellowish with black stripes on the tops of the femoral segments (third-fifth instars), rarely completely black (third-fifth instars). The abdomen is light, the same color as the light areas of the head and thorax. Evaporation sites and parasegmental spots are brown (first instar) or black (second-fifth instars); in older larvae, they are often slightly blurry. The evaporating sites in younger larvae are surrounded by a wide reddish orange ring; in the following stages, the color of this ring more or less fades. Along the inner edge of the abdominal rim from above and below, there is a wide reddish ocher stripe (second and third instars) or ocher stripe (fourth and fifth instars), sometimes weakly expressed (fourth and fifth instars). The sternite spots in the middle of the lower surface of the abdomen are wide; in the older stages, they are often faded so that only thin strips remain at the front edges of the sternites. The top and bottom of the abdomen are sparsely dotted with small light and black dots (third-fifth instars) small dots; often all dots are light and poorly visible (Shrejner, 1915).

Fig 5: E. ornata imagoes and larvae at the Training, Research, and Production Center Doslidne Pole (Experimental Field of V.V. Dokuchaev KhNAU, 2007).
Range
This bug is found throughout Ukraine, in Moldova; to the north, its range reaches Belarus, the Bryansk, Ivanovo, Gorky, and Sverdlovsk Regions. It was also seen in Central Asia and Siberia. ID Shapiro (Shapiro, 1951b) believed that E. ornata was more southern than E. ventralis, with the northern border of its range at 52, 55° north latitude. This border passes through Warsaw, Bryansk, Kaluga, Ryazan, Kuibyshev, Orenburg, and Kustanay, but they are places where E. ornata was found relatively frequently. E. ornata is found in western Europe (to the south of Poland), North Africa, Asia Minor, Pakistan, Kashmir, and China. Within its range, in particular in the forest-steppe and western Ukraine, E. ornata is distributed quite evenly, not patchily like E. ventralis, although it is more numerous and inflicts more damage in the lower Volga Region (from Sarepta and below) and in the Fore-Caucasus (in Krasnodar and Stavropol Territories, Rostov Region) (Porchinskij, 1909; Shogolev and Strukova, 1932; Lutikov and Zhilin, 1935; Leston, 1955).
Biology and ecology. The time and type of egg laying, fertility, and developmental duration of larva instars in E. ornata are the same as in E. ventralis. In the northern part of its range, it has one generation per year, and in the south-two generations, even in the south of the Krasnodar Territory. However, some researchers, for example, NL Saharov (Saharov, 1934), claimed that in the Lower Volga region this bug could have three generations per year.
Under natural conditions, E. ornatalives in all plants mentioned for E. ventralis, as well as many other wild crucifers (Vilna and Bondar, 2013, Vilna, Yevtushenko and Stankevich, 2015): Chorisrora tenella, Arabis (rockcress), Barbarea (winter cress), Sisymbrium irio (London rocket), Erysimum causpidatum, Sisymbrium altissimum (tall tumblemustard), S. polymorphum, Lepidium perfoliatum (clasping pepperweed). In Turkey, E. ornatainhabits Iberis sempervirens (evergreen candytuft), Erysimum kotschyanum (Alpine wallflower), E. smyrnaeum, and in western Europe, Cardamine amara (large bitter-cress) and C. pratensis (cuckoo flower). It is not associated with cabbage as closely as the previous species. E. ornata is more xerophilous, so it is common in various habitats and more common in plants that do not belong to Brassicaceae. Therefore, young imagoes and older larvae were sometimes observed feeding on alfalfa, Gossypium, cereals, as well as on various wild plants. GM Roshko (Roshko, 1953) even believed that E. ornata lived on Umbelliferae plants, with which I cannot agree.
Economic significance
E. ornata was noted as a cabbage pest in all areas typical of E. ventralis and often inflicted significant damage on this crop. It often and in large numbers also appeared in cabbage, radish, turnips seed, as well as in oilseed crops (mustard, more seldom in false flax, rapeseed) (Loginova et al., 1979) and, according to YeV Kucherov’s data (Kucherov, 1951), in colewort. E. ornata sometimes causes significant damage. According to ID Shapiro’s data (Shapiro, 1951b), when five bugs feed on one mustard plant (from the onset of anthesis), seeds were not formed at all, and when they feed on mustard from the beginning of pod formation, seed yield decreased by 67% compared to control. These seeds turned out to be 90% shriveled (only about 5-6% of the control seeds were shriveled), and their germinability decreased to 54% (the germinability of the control seeds was 97.5%). The enemies of E. ornata are the same species that have been recited for E. ventralis (Saharov, 1934; Suhorukov, 1953).
Rape bug (Eurydema oleracea L.)
The imago differs from other species by its smaller size, dark main color of the body (black with a greenish or bluish sheen) (Fig. 6) and a short head, strongly concave on the sides in front of the eyes (Bogoyavlenskaya, 1915; Budazhapov, 1993). The head is black, bordered on the outer edge of the parafacialia with a light (red, yellow, or brownish) rib, evenly elevated along the entire length rib, and sometimes has light stripes that converge in front and extend along the inner edges of the parafacialia. The antennae are black (Bogdanov-Katkov, 1920). The pronotum is black with a light, fairly wide stripe along the middle and two stripes on the sides that extend in the direction from the anterior to the lateral angles; in light-colored specimens, the pronotum only has two large dark spots on the sides, sometimes even cut across by a narrow light stripe (Rusanova, 1926). The shield is dark with a light top and spots on the sides. The color of the exocorium at the base is light, but farther to the top it looks like a narrow strip along its outer edge, reaching the top of the outer angle. The rest of the outer apical angle of the corium is always dark (except for f. aberrans Horv.). The clavus and mesocorium are dark, but the mesocorium at the level of the shield top has a light transverse spot. The diaphragm is brownish to black, lighter on the outside.

Fig 6: E. oleracea in the Doslidne Pole of the Training, Research and Production Center(Experimental Field of V.V. Dokuchaev KhNAU, 2014).
The abdominal rim is light with dark spots near the anterior angles of the top and bottom segments. The bottom surface of the abdomen is black to completely light in color, except for black spots around the spiracles. In dark specimens, the legs are black, with a narrow light ring on the legs, at least the hind legs, and in light specimens, the femoral segments are almost completely light, while the tibiae have a wide light ring in the middle. The length is 5.5 7.0 mm. (Rusanova, 1926). The larva is very similar to the E. festiva larvae, but it is smaller. In addition, it can sometimes differ by the features described below. The dark pattern on the body of E. oleracea larvae, compared to E. festiva larvae of corresponding instars, is usually more developed (Polivanova, 1956). The posterior edge of the pronotum is often not completely light and less rounded in a roller-like manner. The surface and apical part of the parafacialia are wrinkled (in E. festiva, the wrinkles on them are indistinct or absent at all). The abdomen is dotted with large dots at the top and bottom, and the number of black dots in E. oleracea is usually much larger than in E. festiva (Hlebnikova, 1923).
Range
The rape bug can be found throughout Ukraine and Moldova; to the north, its range reaches 62-64° north. Latitude; this species is common in Siberia, relatively rare in Central Asia (Fig. 7), and very common in the Middle East, North Africa, and western Europe (Porchinskij, 1909).
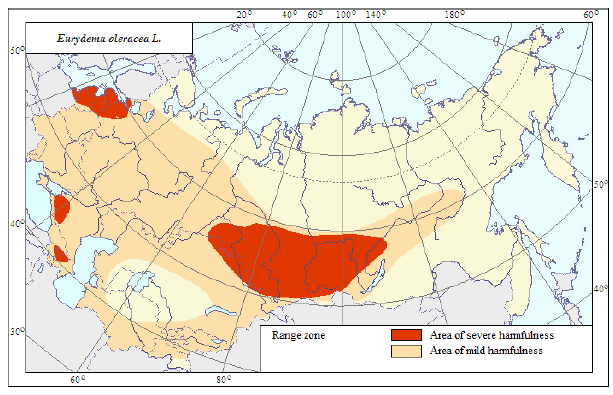
Fig 7: Range zone and areas of harmfulness of the rape bug.
Ecology
In Ukraine, the rape bug is very common in various habitats. In the forest-steppe, it lives equally often in open fields and meadows and on forest edges and swards; in the steppe it tends to inhabit the lowlands and river valleys, so SI Medvedev (Medvedev, Treml and Shapiro, 1952) called it a "meadow mesophile." In the Caucasus, the rape bug was found in all areas-subtropical, forest, subalpine, and alpine (Kobahidze, 1957). In the mountains of Central Asia, it lives mainly in the highlands (Kirichenko, 1928), and in the Zakarpatska Oblast, as GM Roshko described (Roshko, 1953; Roshko, 1955), it inhabits mostly flat and hilly places and only comes up in mountain river valleys to altitude of 1000 m above sea level. However, studying the hemipteran fauna in Bukovina and Galicia (Chernivetska, Stanislavska, Drohobytska and Lvivska Oblasts region), Stobiecki (Stobiecki, 1915) wrote that the rape bug was common everywhere and climbed up mountains to the mountain pine zone. In Switzerland, according to Hoffmänner’s (Hoffmanner, 1925) and other research data, it reaches an altitude of 1700 m and even 2600 m.
The rape bug lives on many crucifers (Vilna and Bondar, 2013, Vilna, Yevtushenko and Stankevich, 2015), in particular on Chorispora tenella (purple mustard), ardamne (bittercress), Arabis (rockcress), Nasturtium officinale (watercress), Barbarea vulgaris and. arcuata(bittercress), Erysimu m (wallflower), Alliaria officinalis (garlic mustard), Descurainia Sophia (flixweed), Arabidopsis t haliana (thale cress), Sisymbrium altissimum (tall tumblemustard), S officinale (hedge mustard), S. volgense, S. l oeselii (small tumbleweed mustard) (and other Sisymbriumspecies), Hesperis matronalis (dame's rocket), Armoracia rusticana (horseradish), Berteroa incana (hoary alyssum), Draba (whitlow-grasses), Camelina microcarpa (littlepod false flax), Thlaspi arvense (field pennycress), Capselia bursa pastoris (shepherd's purse), Lepidium draba (whitetop), L. c ampestre (field pepperwort), Brassica campestris, Sinapis arvensis (charlock mustard), Crambe (colewort) and others; some of these plants were mentioned as food for the rape bug in the Tomsk Region (Hlebnikova, 1923) and Krasnodarsk Territory (Shapiro, 1951b). In the West, food plants for these species include Brassica (cabbage), Sinapis (mustard), Erysimum (wallflower), Cochlearia (scurvygrass), Biscutella, Stenophragma phulion, Cardamine amara (large bitter-cress). Ziarkiewicz (Ziarkiewicz, 1953) particularized 16 food plants for these species in Poland, where, in addition to the above-listed, the rape bug can eat Diplotaxis tenuifolia (wild rocket), Matthiola incana (common stock), Roripa silvestris(yellow field cress), Sinapis alba (white mustard), Raphanus raphanistrum(wild radish), R. s ativus (radish). Our special experiments lead to the common opinion in the literature that the rape bug feeds on Umbelliferae plants.
In addition to cruciferous plants, the rape bug, according to published data, also feeds on stinging nettle, wormwood, thistle, lamb's quarters, hemp, and even on couch grass. MI Khlebnikov (Hlebnikova, 1923) also mentioned cardiac motherwort, and Butler (Butler, 1923) wrote about frequent findings of adult bugs on burnet-saxifrage (Pimpinella saxifraga). Guide (Guide, 1921) found E. oleracea larvae on flax. However, ID Shapiro (Shapiro, 1951b), founding experiments on the survival of larvae and life span of imagoes of E. ventralis, E. ornata, and E. oleracea (feeding on different plant species of ten families), concluded that only crucifers and garden nasturtium were edible plants for bugs. ID Shapiro considered all published data on the relationships of the species with other plants as a result of the registration of accidental visits of Eurydema bugs to these plants. Completely sharing the first part of ID Shapiro’s conclusion, I cannot agree completely with the second one. Indeed, all of these species are oligophagous, trophically related almost exclusively to Brassicaceae, and Eurydema bugs never reproduce their kind in habitats lacking Brassicaceae. However, I observed that older larvae and young imagoes of E. oleracea (sometimes of E. ornata, rarely of E. ventralis) willingly fed on plants of other families, even Gramineae (especially on their seeds) in the course of fattening feeding. Obviously, some of the evidence in the literature is applicable to the fattening period, for example, persistently emphasized by AN Melnichenko (Melnichenko, 1949) harmful activity of Eurydema bugs in rye, wheat, and sunflower fields in the steppe trans-Volga region.
Biology
Rape bug overwinters in plant litter in abandoned fields, weedy areas of cultivated fields, and roadsides, as well as under fallen leaves among shrubs and trees (Lisovij, 1999; Vilna, 2014a). Bugs awaken immediately after snow melting away, simultaneously with Bishop's Mitre (Aelia acuminata). In early spring, bugs are sometimes found in coniferous and deciduous trees, for example, in pine shoots and willow flowers, but soon they gather in groups (20-30 specimens) in individual plants of Erysimum (wallflower), Sisymbrium, Thlaspi (pennycress), etc., where they feed and mate, and when severely damaged plants die, the whole group moves to near plants. The feeding-maturity period lasts about 10-20 days, depending on weather. Before laying eggs, the bugs settle fairly evenly in different habitats where their food plants grow.
In the southern part of the range (Crimea, Fore-Caucasus, and Lower Volga Region), the rape bug begins to lay eggs in late April; in the forest-steppe-in mid-May; and in the forest zone-in early June. Females lay eggs on any aboveground parts of food plants or on growing nearby non-food plants, less often on plant remains, and sometimes on ground lumps. The placement and number of eggs are the same as those of the cabbage bug, but the fertility of females is lower (according to MI Hlebnikova data (Hlebnikova, 1923, Hlebnikova, 1927), about 60 eggs). The development of eggs lasts about 5 to 8 days (at the average daily temperature of 20 to 23°C) and up to 17 to 19 days (at 12 to 13°C). Larvae develop for 32 to 39 days, on average for approximately 35 days at 20-26°C; the development of the first instar lasts 3 to 5 days, of the second instar-5 to 8 days, of the third instar-6 to 10 days, of the fourth instar-8 to 12 days, and of the fifth instar-10 to 14 days (Puchkova and Puchkov, 1958). In the forest-steppe, the rape bug finishes laying eggs in June and, as early as July, bugs of the wintering generation are almost undetectable. However, in Siberia, the rape bug may have a second (though incomplete) generation, which was seen in the Yakutsk region.
The winging of young imagoes of the first generation in the Voronezh Region, Poltavska and Kyivska Oblasts begins in late June and comes en masse in mid-July. In the southern part of the range, young bugs appear one or two weeks earlier, and in the forest zone one or two weeks later. Shortly after winging, females begin to lay eggs, and in July-August the second generation of the pest develops. Generations of the rape bug overlap and boundaries between them are difficult to draw. In the forest-steppe, young bugs of the second generation begin to appear in mid-August, while in the Krasnodar Territory and the Lower Volga Region, in early August. The larvae of the younger and middle instars of both generations feed mainly on young Brassicaceae shoots, but the older instars feed mainly on ripening seeds (Shogolev, 1923; Stankevich et al., 2010; Stankevich and Vilna, 2012a; 2012b; Vilna, 2014b; 2013c; 2013d; 2015; Yevtushenko et al., 2015). At the end of July in the Poltavska Oblast, there are large populations of this species in the fields after the harvest of cereals and after finishing the fruitification and drying plants of Camelina microcarpa (littlepod false flax) and Arabidopsis thaliana (thale cress). The migration of older larvae of the first generation from Brassicaceae to plants of other families is not very active. The migration markedly intensifies during the first-generation imago flight and is more often intense for the second-generation older larvae and imagoes (as well as for the first-generation specimens with delayed development), when most Brassicaceae plants, on which the rape bug feeds, dry up. Because of this, in the second half of summer, one can often see the rape bug feeding on wormwood, thistle, nettle, Pimpinella, cereals with ripe ears, as well as on other noncruciferous plants and agricultural crops. On these plants, bugs are only found singly, and these plants cannot be considered food for this species (Kuznecov, 1932). Adult bugs of the last generation are found in plants until late fall, although their migration to overwintering shelters begins in the second half of August (Vilna, 2013c; 2014a).
Economic significance
The rape bug damages different varieties of cabbage, rapeseed, radish, rutabaga, turnip, white turnip, mustard, false flax, horseradish, and colewort, as well as some non-cruciferous crops-lettuce, asparagus, sugar beet, Jerusalem artichokes, Kazakh dandelion, sunflower, rye, and wheat. In western Europe, harmful activities of the rape bug (in addition to cruciferous plants) were observed on potato, rye, wheat, oat, barley, garden, and ornamental plants. However, the rape bug only inflicts significant damage on Brassicaceae crops (Vovk, 1920).
Parasites and predators
Trissolcus simoni Mayr. And Microphanurus vassilievi Mayr. were defined as parasites of E. oleracea eggs, and Clytomyia catinua Pz. and Eurythmelus goochi parasitize imagoes and larvae. However, the latter species is a specialized parasite of capsid bugs (family Capsidae), and the statement that it is a parasite of the rape bug requires confirmation. In addition, larvae of the pest are exterminated by predatory bugs (Nabis ferus L., N. apterus L., Prostemma aeneicolle Stein., Ceocoris ater L.), spiders, and ants (Suhorukov, 1953).
Protection measures against Eurydema bugs
Information on protection measures against Eurydema bugs has been available since the nineteenth century. Harmful activities of the cabbage bug in the Russian Empire were first noted in 1861, when the bug completely ate up planted cabbage in the Khvalynsky Uyezd of the Saratov Governorate. According to YaF Schreiner’s data (Shrejner, 1915), Eurydema bugs have been widespread since 1914 in the Astrakhan Governorate, the Caucasus, the South Caucasus, northern and central Russia, Siberia, the Volga region in the Samara Governorate, and the Saratov and Sukhumi Regions. They damaged mustard and cabbage plantations. The author noted that, to control the number of bugs, bug-inhabited plants were sprayed with a Quassia or tobacco decoction. However, such spraying was effective only against wingless and sluggish larvae, but the imagoes immediately fled as soon as they felt drops of fluid on their bodies. Since bugs gathered in groups, he recommended more effective methods: sweeping with a net and sprinkling plants with ash, lime dust, or with crushed bird or horse manure during the growing period (Shtejnberg, 1907). F. Keppen (Keppen, 1883) recommended filling plant interrows and horse manure with its subsequent burning as an effective measure.
According to NL Sakharov’s data (Saharov, 1930), to control the numbers of Eurydema bugs, rapeseed was sprinkled with calcium arsenate at the Saratov Experimental Station in 1928. Sprinkled rapeseed yielded a yield of 6.77 cwt/ha versus 1.56 cwt/ha from unsprinkled rapeseed (or 334% less). In 1929, 4.10 cwt/ha of seeds were harvested from sprinkled crops, while the losses in the unsprinkled fields amounted to almost 100%. In the 1920s-1930s, recommendations on the control of Eurydema bug and rape pollen beetle numbers were as follows: spraying crops with sodium arsenate (400 g of sodium arsenate and 1,200 g of quicklime per 480 liters of water) or with copper acetoarsenite (400 g of copper acetoarsenite and 1,200 g of quicklime per 360-400 liters of water); sprinkling crops with calcium arsenate; extirpation of cruciferous weeds and sowing within optimal timeframes (Shogolev, 1923). In the 1930s, VM Schogolev (Shogolev and Strukova, 1932) also recommended spraying with barium chlorate, copper acetoarsenite, and sodium silicofluoride. He also recommended sprinkling. It was carried out with horse, hand and aviation sprinklers using anabadust, nicotine sulphate dust, or tobacco dust mixed with lime and calcium arsenate. Furthermore, the eradication of cruciferous weeds was recommended. In the 1940s, sprinkling crops with mixtures of tobacco dust and lime, pyrethrum and ash, ash with kerosene or kreoline, and calcium arsenate was recommended. Weeding and early planting were mandatory (Moric-Romanova et al., 1941); marginal spraying and attracting birds were also recommended (Saharov, 1934).
In the 1950s, preanthesis sprinkling with hexachlorane, dichlorodiphenyltrichloroethane (DDT), nicotine sulfate dust, calcium arsenate, and tobacco dust was recommended to protect crops against Eurydema bugs. Great attention was paid to agronomic measures (weeding, removing fallen fruits/seeds, early sowing) (Dobrovolskij, 1950; Velichko, 1951; Kosov et al., 1952). In the 1960s, according to the Puchkov data, measures to control the numbers of Eurydema bugs were the following: eradication of wild crucifers growing near cultivated cruciferous plants; early planting of cabbage seedlings and providing all the conditions for their rapid development; late planting of cabbage with beans and tomatoes as cover crops (recommended for the Krasnodar Territory by Suhorukov (Suhorukov, 1953); sprinkling plants inhabited by larvae and adult bugs with hexachlorane powder (15 25 kg/ha) and DDT (30-40 kg/ha) or with a mixture of these insecticides in a ratio of 2:1 (15 kg/ha) (Alimbekova et al., 1949; Bardysheva, 1967; Narzikulov, 1968). During the season, the crops were sprinkled twice or four times, depending on the time of the emergence of the pest. For sprinkling cabbage, it is better to use only DDT before head setting (Suhorukov, 1947, Volovik, 1953, Suhorukov, 1953; Leshenko, 1956; Gerasimov and Osnickaya, 1961); to manually collect and destruct egg clutches, larvae, and imagoes (Korolkov, 1929; Agapova, 1953); to reproduce and release eggs of the parasitoid Trissolcus (Suhorukov, 1947; Suhorukov, 1953).
As the Regional Plant Protection Station in the Chita Region reported, white turnip grown on an area of 122 hectares on a collective farm in the Karymskoye District, as well as cabbage, radish, and white turnip grown on an area of 115 hectares on the state farms Shilkinskiy and Mitrofaninskiy and on the collective farm named after Kirov in the Shilka District were severely damaged by Eurydema bugs in 1970 and 1972. In 1978-1979, Eurydema bugs caused considerable damage to cabbage and other crucifers in the Petrovsk-Zabaykalskiy, Krasnyy Chikoy, Aksha, Kirensk, Chernyshevsk and Shelopugino districts. At the same time, Brassicaceae plants grown on an area of 30 hectares on a collective farm in the Petrovs-Zabaykalsk district and on collective farms in the Krasnyy Chikoy and Aksha districts, where bugs populated 25% to 60% of plants with an imago density of up to 5 specimens (maximum 8) per plant, were especially severely damaged. The situation was almost the same in the period 1981 to 1982. In these areas, the percentage of damaged plants was 60%, with the average density of Eurydema bugs of up to 4 specimens (maximum 8-10) per plant. The economic threshold of harmfulness was more than 2 bugs per plant in cabbage at the beginning of head formation and 1-2 bugs per m2 in root vegetable sprouts (Budazhapov, 1993). Thus, in the 1970s and 1980s, the following protection measures were recommended (Kosmodemyanskij and Kulik, 1967; Gorodnij, 1970): agrotechnical measures (regular eradication of cruciferous weeds in greenhouses, where bugs that overwintered throng; high farming techniques for the cultivation of cruciferous crops; application of phosphorus-potassium fertilizers; and soil loosening between rows) increase the resistance of plants to bugs, while chemicals kill pests (results of surveys demonstrated that, if the density of Eurydema bugs on food root vegetables exceeded 2 specimens per plant, crops should be sprayed with 80% chlorophos WP (0.8 kg/ha) or with malathion, 40% EC (0.6 L/ha) (Budazhapov, 1993). Since the 1990s, integrated protection has been recommended, including the choice of sowing sites and forecrops, crop rotation, and spatial isolation. Agrotechnical measures play an important role. For spring rape, the type of tillage depends on the forecrops. Stubble breaking and dragging are mandatory. High-quality seeds, seed preparation for sowing, fertilizers, optimal sowing timeframes, and crop maintenance are important. Anti-bug spraying crops with insecticides deltamethrin, 25% CE (0.3 L/ha) and phosalone, 35% CE (1.5-2 L/ha) is recommended in the 4-6 leaf phase before budding (Lisovij, 1999). Since cruciferous crops began to occupy large areas, the issues of high yields and their protection against pests and diseases have become burning. Despite the huge role played by organizational, economic, agronomic, and biological methods, they are often unable to reduce the density of pests or the development of diseases below the economic threshold of harmfulness. The chemical method is the most eradicative in the control of Eurydema bugs. As the importance of rapeseed as an oil crop increases, the assortment of agents that regulate their numbers expands. Pre-sowing seed treatment with systemic insecticides is becoming widespread. This allows for reduction in doses of active substances, pesticide load, and salary costs (Dovgan and Kozak, 2006; Fedorenko et al., 2010; Stankevich and Vilna, 2012b; 2014a; Vilna and Stankevich, 2013; 2014).
The vast majority of modern authors (Mazur et al., 1997; Nikitchin et al., 1997; Strukova, 1999; Satubaldin, 2004; Laba and Sitnik, 2006; Sekun et al., 2008; Sitnik, 2008) argue that today the protection of rapeseed fields against Eurydema bugs is impossible without chemicals, but only if their application is environmentally and economically sound. Since the twentieth century, preference has been given to less toxic agents applied in low doses. The assortment of insecticides recommended for the protection of Brassicaceae oilseed crops against Eurydema bugs is very wide and it is not necessary to emphasize any specific agents. To develop effective measures to protect cruciferous crops against Eurydema bugs, one should take into account the dependence of pest populations on weather factors. Reduction in insecticide doses and in the overall pesticide load can be executed through treating field edges (Yevtushenko et al., 2008). To control the number of Eurydema bugs in spring rape during stem formation, it is recommended to use insecticides 5% imidacloprid WP and 20% imidacloprid SC. Spraying with systemic agents is especially advisable, because they are more effective than contact agents and their effects are longer and less dependent on weather factors (Mazur et al., 1997; Kiforuk, 1998; Yevtushenko et al., 2009). Eliminating cruciferous weeds is mandatory in Brassicaceae crop fields (Iversen, 1883; Vilna, 2013c; 2013d; Vilna et al., 2015).
Organizational, economic and agrotechnical measures are essential in limiting the number of rapeseed pests. For example, rapeseed in crop rotation should be sown after forecrops, which are harvested early, such as potato and cereals. At the same time, it is necessary to adhere to the spatial isolation requirements, keeping the distance of 1 km between current and previous year's fields of domestic crucifers. Rapeseed should not be sown in the same field after Brassicaceae crops. Managing weeds on a timely basis in all fields throughout crop rotation ensures the eradication of wild Brassicaceae species, which are reservoirs for the reproduction of rapeseed pests. Rapeseed should be sown within the optimal timeframe; spring rapeseed should not be sown too late, as seedlings are very vulnerable to being easily damaged by flea beetles and other pests. To protect crops against E. oleracea (rape bug), Meligethes aeneus (rape pollen beetle), Entomoscelis adonidis, Athalia rosae (turnip sawfly), Ceuthorhynchus quadridens, Ceutorhynchus obstrictus, and Pieris brassicae (cabbage butterfly) during the phenophase ‘4-6 leaves-budding onset’, crops are treated with beta-cyfluthrin, EC (3 L/ha), deltamethrin 25 WG (0.07 kg/ha), deltamethrin, EC (0.250.5 L/ha), chlorpyrifos EC (0.50.6 L/ha), phosalone 35 EC (1.5 2 L/ha), imidacloprid WG (0.07 kg/ha), thiacloprid (Calypso) 480 SC (0.2 L/ha), lambda-cyhalothrin 050 CS (0.15 L/ha), acetamiprid SP (0.10-0.12 kg/ha), chlorpyriphos 25 CS (0.75-1.0 L/ha), chlorpyriphos+biphenthrin 420, EC (0.40-0.75 L/ha), bifenthrin+alpha-cypermethrin (Fury), EW (0.1 L/ha), alpha-cypermethrin (Fastac) EC (0.1-0.15 L/ha), alpha-cypermethrin (Caesar), EC (0.125-0.15 L/ha), chlorpyriphos+cypermethrin, EC (0.5-0.6 L/ha), deltamethrin, EC (0.3 L/ha) (Perelik pesticidiv..., 2012).
The use of pesticides is allowed on rapeseed intended for technical and seed needs, but it is forbidden to use rapeseed oil for food, as well as straw and oil meal for feed, if fields were treated with deltamethrin, phosalone, or alpha-cypermethrin (Fedorenko et al., 2013). From our studies, it is also known that the imagoes of overwintered bugs first inhabit wild crucifers (Vilna, 2013c, Vilna, 2014a, Vilna, Yevtushenko and Stankevich, 2015), then white cabbage seed plants (Vilna, 2013b, Vilna and Stankevich, 2013, Yevtushenko and Vilna, 2014a), and later spring rapeseed and mustard fields (Fedorenko et al., 2010, Teslina and Stankevich, 2010, Vilna and Stankevich, 2012, Yevtushenko et al., 2012a, Stankevich and Vilna, 2012c, Stankevich and Vilna, 2012d). Initially, they inhabit the fields around the perimeter (Yevtushenko and Vilna, 2014a). In overwintering places, the highest density of Eurydema bugs is recorded in forest belts, on shoulders of roads and highways; there are fewer Eurydema bugs at forest edges (Yevtushenko, Vilna and Stankevich, 2015).
According to Gorbatko’s recommendations (Gorbatko, 2010), it is necessary to perform high-quality tillage, fertilize with N60P55K30, plant resistant varieties and treat the 120-meter margins of rapeseed fields instead of whole fields. According to the data from VS Zhuravskij (Zhuravskij, 2012), Eurydema bugs begin to cause damage during the ‘rosette formation-stem formation’ phenophases. Phytosanitarily, winter wheat, early cereals, and potato are the best forecrops for spring rapeseed. Early return of rapeseed to the same field contributes to a rapid increase in the number of ceutorrhynchid beetles. To significantly improve the phytosanitary condition of spring rapeseed fields, the spatial isolation requirements must be met, keeping the distance of 1 km between the current rapeseed fields and the fields of domestic crucifers of the previous year. Upon harvest, the laying of the rapeseed swath is started during the yellow-green ripeness phase with a water content in the seeds of 30 35%. The cutting height is 15-20 cm. Seeds are threshed at a water content of 12%. Direct combining starts with a water content in seeds of 12%. Timely and short harvest causes the death of significant numbers of eggs and larvae that have not completed development (Fedorenko et al., 2015). Annual and perennial weeds, as well as sprouts from fallen seeds, are reservoirs for most pests, especially Eurydema bugs (Vilna and Stankevich, 2012; Vilna et al., 2015; Fedorenko et al., 2015).
In the Kharkivska Oblast 2014 forecast, the State Phytosanitary Inspectorate of the Kharkivska Oblast indicated that in 2013 Eurydema bugs inhabited 13% of the area under winter oileed rape, damaging 6.9%, maximum 10%, of plants with the average density of 2.2 specimens, maximum 6 specimens, per square meter. To protect winter rapeseed fields, organizational, economic, and agrotechnical measures were recommended. Additionally, in September-October (2-4 leaves-formation of rosettes), it was recommended to spray fields against Eurydema bugs beta-cyfluthrin, EC (0.3 L/ha); deltamethrin, WG (0.07 kg/ha); phosalone, EC (1.5 2 L/ha). When spraying with phosalone, straw is prohibited for animal feed and oil for food (Prognoz 2014). The forecast for 2015 stated that Eurydema bugs inhabited rapeseed fields during the stem formation phase-‘blooming onset’ with an average bug density of 2.0 specimens/sq m, which was slightly less than in 2013 (2.2 specimens/m2). Under favorable conditions, one could expect a high level of bug damage in 2015, especially in fields adjacent to their overwintering housings. Organizational, economic and agrotechnical measures were also recommended: spraying of vegetative weeds with herbicides, spraying winter rape with insecticides deltamethrin 25 WG, (0.07 kg/ha), phosalone 35 EC (1.5-2 L/ha) during the phenophase ‘2-4 leaves-rosette formation’ (Prognoz....., 2015). Our studies demonstrated that one of the necessary and effective protection measures was spraying spring rape and mustard fields with systemic insecticide 24% thiacloprid OD at a dose of 0.25 L/ha during the yellow bud phenophase, but before anthesis (Yevtushenko et al., 2009; Stankevich, 2013; Vilna and Stankevich, 2013; Yevtushenko et al., 2014a; Yevtushenko and Vilna, 2014a; Yevtushenko et al., 2015; Stankevich and Vilna, 2014b).
To protect spring rapeseed and mustard fields against Eurydema bugs, it is also proposed to spray plants with systemic insecticides 25% thiacloprid OD, imidacloprid, SC, 20% acetamiprid SP, chlorpyriphos+cypermethrin, 500 EC during the yellow bud phenophase (Yevtushenko et al., 2009; Stankevich and Vilna, 2014b).
According to the list of pesticides and agrochemicals approved for use in Ukraine in 2012 (Perelik pesticidiv..., 2012) to protect oilseed crops against Eurydema bugs, it was recommended: deltamethrin, EC (0.25 0.5 L/ha), phosalone 35, EC (1.5 2 L/ha), thiacloprid SC, SC (0.15/8L/ha), imidacloprid+lambda-cyhalothrin, WG (0.05 0.07 kg/ha), lambda-cyhalothrin 050 CS (0.15 L/ha), lambda-cyhalothrin CS (0.15 L/ha), acetamiprid, SP (0.10 0.12 kg/ha), cypermethrin+chlorpyriphos, EC (0.6 L/ha), imidacloprid+lambda-cyhalothrin, SC (0.05 L/ha), alpha-cypermethrin, EC (0.1-0.15 L/ha), chlorpyriphos+cypermethrin, EC (0.6 L/ha), dimethoate, EC (1.2 L/ha) for rape seed; chlorpyriphos+cypermethrin, EC (0.5-0.6 L/ha) for mustard for spraying during the growing period.
Conclusion
The analysis of the literary data indicates that despite the considerable number of literary sources devoted to the Eurydema bugs, theirs is still a number of its biological and ecological features which are in close connection with the protection measures for controlling it and these measures have not yet been completely clarified.
Modern systems of plant protection consist in developing and implementing the integrated measures that preserve the crops from the harmful organisms while being the safest for the environment, animals, and humans.
The transition to such integrated systems involves the application of a biological method of pest control, reducing the number of pesticide treatments, the ability to use the preparations of selective action together with the entomophages, etc. An important reserve in this program is the activation and use of natural resources of the beneficial insects (parasitoids and predators) that limit the number of harmful insect-phytophages.
References
Agapova, K.V. (1953). Nekotorye dannye o prichinah kolebaniya chislennosti kapustnogo klopa (Eurydema ornatam L.). Rostov State University, 2:43-45 (in Russian).
Alimbekova, M.G., Kukshina, E.P., Nikitina, T.F. (1949). Glavnejshie vrediteli, bolezni i sornyaki selskohozyajstvennyh kultur Gorkovskoj oblasti i mery borby s nimi. Gorkij: OGIZ (in Russian).
Arnoldi, K.V. (1947). Vrednaya cherepashka Eurygaster integriceps Put. v dikoj prirode Srednej Azii v svyazi s ekologicheskimi i biocenologicheskimi momentami ee biologii. Sbornik Vrednaya cherepashka, I, 136-269 (in Russian).
Arnoldi, K.V. (1952). K vyyasneniyu zonalnyh zakonomernostej obrazovaniya novyh gruppirovok nasekomyh i zaseleniya lesoposadok kserofilnymi vidami pri stepnom lesorazvedenii. Zool. Zhurn, XXXI(3), 329-346 (in Russian).
Arnoldi, K.V. (1957). O teorii areala v svyazi s ekologiej i proishozhdeniem vidovyh populyacij. Zool Zhurn.T., pp:1609-1629 (in Russian).
Bardysheva, A.M. (1967). Vrediteli i bolezni selskohozyajstvennyh rastenij i mery borby s nimi v usloviyah Magadanskoj oblasti. Magadan: Magadan Press (in Russian).
Bej-Bienko, G.Ya. (1930). K voprosu o zonalno-ekologicheskom raspredelenii saranchevyh v Zapadno-Sibirskoj i Zajsanskoj nizmennostyah. Tr. po zash. Rast, I, (in Russian).
Belanovskij, I.D. (1951). Tahiny USSR. Ch. I. Kiev (in Russian).
Belanovskij, I.D. (1953). Tahiny USSR. Ch. II. Kiev (in Russian).
Bodrenkov, G.E. (1927). Materialy po faune Hemiptera-Heteroptera Smolenskoj gubernii. Tr. Smolensk. ob-va estestvoisp. i vrachej, II, pp:3-16 (in Russian).
Bogachev, A.V. (1941). Zoologicheskie nablyudeniya v Milskoj stepi, Hemiptera. Izv. Azerb. fil. AN SSSR, 1:53-60 (in Russian).
Bogdanov-Katkov, N.N. (1920). Prigotovlenie i primenenie glavnejshih sostavov, upotreblyaemyh dlya unichtozheniya ogorodnyh nasekomyh. Peterburg: Gosizdat (in Russian).
Bogoyavlenskaya, M.G. (1915). K biologii Naltica oleracea L. Otchet o deyat. miko-entom. op. st. Vseros. ob-va saharozavodov za 1914 god, pp:51-74 (in Russian).
Bondarovich, M.Ya. (1926). Vrediteli i povrezhdeniya ogorodnyh kultur. Vredit. i povrezhdeniya v 1926 godu pod red. Rahmaninova, pp:38-49 (in Russian).
Bramson, K.L. (1894). Vrednye nasekomye. Ch. I, pp:87-88 (in Russian).
Budazhapov, V.C. (1993). Zashita rastenij ot vreditelej v Zabajkale. Ulan-Ude, Buryat. kn. izd-vo, pp:307-310 (in Russian).
Butler, E.A. (1923). A biology of the British Hemiptera-Heteroptera.
Carayon, J. (1949). Communication l’octheque d’Hemipteres Plataspides de PAfrique tropicale. Bulletin Social Entomology France, 54:66-69.
Carayon, J. (1952). Les mechanismes de transmission hereditaire des endosym-bionts chez les insects. Tijds Entomology, 95:111-142.
Dobrovolskij, B.V. (1950). Vrediteli polevyh kulutr. Rostov na Donu, Rostizdat, pp:132 (in Russian).
Dovgan, S. and Kozak, G. (2006). Tehnologiya-zaporuka uspihu viroshuvannya ripaka. Propoziciya, 11:88-93 (in Ukrainian).
Dupuis, Ch. (1949). On the «late melanin» of the larval stages of Pentatomidae (Hem., Het.). Entomology, 85:229-231.
Fasulati, K.K. (1954). Bioticheskie otnosheniya Hemiptera-Heteroptera v usloviyah celinnoj stepi i kulturnyh polej yuzhnogo Zadneprovya. Nauchn. zap. Uzhgor. gos. un-ta, X, pp:93-104 (in Russian).
Fedorenko, N.V., Stankevich, S.V., Teslina, V.V. (2010). Zahist girchici biloyi na doslidnomu poli HNAU im. V.V. Dokuchayeva. Ekologizaciya stalogo rozvitku agrosferi i noosferna perspektiva informacijnogo suspilstva. Materiali mizhnar. nauk. konf. stud., asp. i mol. uchenih (HNAU, 4-5.10.2010), pp:110-111 (in Ukrainian).
Fedorenko, V. (2014). Shkidniki girchici. The Ukrainian Farmer, 6:60-63 (in Ukrainian).
Fedorenko, V.P. (2012). Strategiya i taktika zahistu roslin. Strategiya. Kyiv, Alfa-steviya (in Ukrainian).
Fedorenko, V.P., Pokozij, J.T., Krut, M.V. (2013). Entomologiya: pidruchnik. Kyiv, Feniks, Kolobig (in Ukrainian).
Flemion, F., Miller, L.P., Weed, R.M. (1952). An estimate of the quantity of oral secretion deposited by Lygus when feeding on bean tissue. Contrib. Boyce Thompson Inst, 16:429-433.
Gerasimov, B.A., Osnickaya, E.A. (1961). Vrediteli i bolezni ovoshnyh kultur. 4-e izd. Moscow, Selhozgiz (in Russian).
Giterman, G.E. (1931). Materialy k faune Hemiptera BSSR. Materialy k izucheniyu flory i fauny Belorussii: sbornik, VI, pp:77-104 (in Russian).
Gorbatko, K.A. (2010). Zashita rapsa ot vreditelej v zone neustojchivogo uvlazhneniya Centralnogo Predkavkazya. Thesis of Doctoral DIsserttion. Moscow (in Russian).
Gorodnij, M.G. (1970). Olijni ta efiroolijni kulturi. Kyiv, Urozhaj (in Ukrainian).
Grinev, Yu.K. (1925). K biologii kapustnogo klopa. Izv. Stavrop. st. zash. Rast, 1:27-32 (in Russian).
Guide, J. (1921). Die Wanzen (Hemiptera-Heteroptera) der Umgebung von Frankfurt am Main und des Mainzer Beckens. Abh Senck Naturf Ges, 37:329-503.
Hlebnikova, M.I. (1923). Sezonnye izmeneniya v okraske kapustnogo klopa (Eurydema oleraceum L.). Izv. Sibirsk. entomol. Byuro, 2:63-65 (in Russian).
Hlebnikova, M.I. (1927). Materialy po biologii rapsovogo klopa v usloviyah Zapadnoj Sibiri. Izv. Tomsk, gos. un-ta, 27:200-208 (in Russian).
Hoffmanner, B. (1925). Beitrage zur kenntnis der Oekologie und Biologie der schweizerischen Hemipteren. Rev Suisse Zoology, 23:181-206.
Iversen, V.Ye. (1883). Vrednyya polevyya nasekomyya. Opyt prakticheskoj entomologii. Sankt-Peterburg, Izd. F. Pavlenkova (in Russian).
Kalyanov, P. (1911). Kapustnyj klop. Selskohoz. listok Kamyshinskogo uezdnogo zemstva, 1:27-29 (in Russian).
Keppen, F. (1883). Vrednye nasekomye. 2:394-406 (in Russian).
Kiforuk, I.M. (1998). Zahist roslin. Ripak, Ivano-Frankivsk, Siversiya LTD, pp:109-153 (in Ukrainian).
Kirichenko, A.N., Talickij, V.I. (1933). Obzor fauny nastoyashih poluzhestkokrylyh s.-v. chasti Donbassa (b. Luganskij okrug). Tr. Zool. in-ta AN SSSR, 1:415-482 (in Russian).
Kirichenko, A.N. (1908). Recenziya na statyu Reuter. Russk Entomol Obozr, 8:337-338 (in Russian).
Kirichenko, A.N. (1915). Fauna Hemiptera-Heteroptera Veliko-Anadolskoj dachi i Mariupolskogo opytn. lesnichestva Ekaterinoslav. gub. Zap. Novoross. ob-va estestvoisp., pp:1-27 (in Russian).
Kirichenko, A.N. (1916). K faune Hemiptera-Heteroptera Kryma. V, Russian Entomology Obozr, 16:87-91 (in Russian).
Kirichenko, A.N. (1925). K faune Hemiptera-Heteroptera Kryma. VI, Russian Entomology Obozr, pp:170-175 (in Russian).
Kirichenko, A.N. (1928). K faune Hemiptera-Heteroptera Kryma. VII, Russian Entomology Obozr, 22:129-132 (in Russian).
Kobahidze, D.N. (1957). Vrednaya entomofauna selskohozyajstvennyh kultur Gruzinskoj SSSR. Tbilisi, Izd-vo AN Gruz. SSR (in Russian).
Korinek, V.V. (1939). Materialy dlya izucheniya fauny poluzhestkokrylyh. Uch. zap. Leningr. gos. un-ta, Ser Biological Nauk, 7:258-283 (in Russian).
Korinek, V.V. (1940). Fauna nekotoryh poluzhestkokrylyh nasekomyh Hoperskogo zapovednika. Tr Hopersk Gos Zapov, 1:174-218 (in Russian).
Korolkov, D.M. (1929). Vrediteli s.-h. rastenij Sochinskogo rajona Chernomorskogo okruga po nablyudeniyam 1926 i 1927. Sochi (in Russian).
Kosmodemyanskij, M.P., Kulik, E.N. (1967). Sareptskya gorchica. Volgograd, Nizhnevolzh. kn. izd-vo (in Russian).
Kosov, N.P., Rameev, H.H., Lopaeva, Z.A. (1952). Maslichnye Kultury Kazan, Tatgosizdat (in Russian).
Kretovich, V.L., Bundel, A.L., Pshenova, K.V. (1943). Proteoliz v zerne, povrezhdennom klopami-cherepashkami. DAN SSSR, pp:35-38 (in Russian).
Kucherov, E.V. (1951). Vrediteli krambe-novoj maslichnoj kultury v Bashkirii. Uch. zap. Bashk Gos Ped in-ta, 3:55-60 (in Russian).
Kuzmina, E.G. (1937). K faune Hemiptera-Heteroptera Centralnogo lesnogo goszapovednika. Tr Centre Lesn Goszapov, 2:209-221 (in Russian).
Kuznecov, V.N. (1932). Klopy. Spisok vrednyh nasekomyh SSSR i sopredelnyh stran. Moscow (in Russian).
Laba, Yu.R., Sitnik, I.D. (2006). Zmina chiselnosti shkidlivih komah pid diyeyu himichnih preparativ na riznih sortah yarogo ripaku. Agrarna Nauka i Osvita, 3:70-73 (in Ukrainian).
Leshenko, A.K. (1956). Olijni ta efiroolijni kulutri. Kyiv, Derzhsilgospvidav URSR (in Ukrainian).
Leston, D. (1955). The British species of carpocoris kolenati (Hem., Pentatomidae). Entamology Mo Mag.
Lisovij, M.P. (1999). Dovidnik iz zahistu roslin. Kyiv, Urozhaj (in Ukrainian).
Loginova, K.M. (1979). Vrediteli kapusty, repy, bryukvy, redisa, redki, turnepsa i drugih ovoshnyh kultur. Opredelitel Selskohozyajstvennyh Vreditelej po Povrezhdeniyam Kulturnyh Rastenij, pp:290-308 (in Russian).
Lutikov, E.I., Zhilin, I.V. (1935). Maslichnye kultury. Moscow, Selhozizdat, p:192 (in Russian).
Masse, A.M. (1954). The hemiptera-heteroptera of Kent. Trans Social Brit Entomology, pp:245-280.
Mazur, I.A. (1997). Nadijnij zahist ripaka ta girchici vid hvorob ta shkidnikiv. Nauk-tehn Byul In-tu Olijnih Kultur, 2:194-196 (in Ukrainian).
Medvedev, S.I. (1954). Osobennosti rasprostraneniya nekotoryh ekologicheskih form nasekomyh v razlichnyh landshaftno-geograficheskih zonah Ukrainy. Zoology Zhurn, 33:1245-1263 (in Russian).
Medvedev, S.I., Treml, A.G., Shapiro, D.S. (1952). Fauna vreditelej agrolesomeliorativnyh pitomnikov v lesostepnoj i stepnoj zonah Ukrainy. Zash Lesonas Ot Vred i Bolezn, pp:47-60 (in Russian).
Melnichenko, A.N. (1949). Polezashitnye lesnye polosy stepnogo Zavolzhya i vozdejstvie ih na razmnozhenie zhivotnyh, poleznyh i vrednyh dlya selskogo hozyajstva. Moscow (in Russian).
Mihajlov, V.K. (1949). Subimaginalnaya faza razvitiya krylatoj stadii i vtorichnaya adapticheskaya pigmentaciya kutikulyarnyh pokrovov u kapustnogo klopa (Eurydsma oleraceum L.). DAN SSSR, pp:877-881 (in Russian).
Moric-Romanova, Z.E., Berezhkov, R.P., Davydov, P.N. (1941). Vrediteli i bolezni selskohozyajstvennyh rastenij Zapadnoj Sibiri i borba s nimi, Novosibirsk, OGIZ (in Russian).
Narzikulov, M. (1968). Vrediteli i bolezni selskohozyajstvennyh kultur Tadzhikistana. Dushanbe, Irfon (in Russian).
Nikitchin, D.I. (1997). Intensivna tehnologiya viroshuvannya ripaka yarogo, girchici i suripici v Ukrayini. Nauk-tehn byul. IOK UAAN, Vip., 2:214-217 (in Ukrainian).
Pachosskij, I.K. (1889). Materialy dlya fauny Hemiptera-Heteroptera yugo-zapadnoj chasti Rossii. Zap. Kiev. ob-va estetstvo-isp., 2:411-420 (in Russian).
Pazhitnova, Z.A. (1952). K poznaniyu nastoyashih poluzhestkokrylyh zapovednika Guralash. Tr. Sredneaz. Universiteta, Vyp Biology Nauki, 2:34-60 (in Russian).
Perelik pesticidiv ta agrohimikativ, dozvolenih do vikoristannya v Ukrayini na 2012 rik (2012). Kiyiv, Yunivest Marketing, p:831 (in Ukrainian).
Polivanova, E.H. (1956). Lichinki glavnejshih rastitelnoyadnyh klopov semejstva Pentatomidae. Zoology Zhurn, 11:1661-1675 (in Russian).
Porchinskij, I.A. (1909). Obzor rasprostraneniya v Rossii vazhnejshih vrednyh zhivotnyh v 1908 godu. Ezhegodnik Gl Upr Zemled, pp:699-709 (in Russian).
Prognoz fitosanitarnogo stanu agrocenoziv, rozpovsyudzhennya karantinnih organizmiv na teritoriyi Harkivskoyi oblasti ta rekomendaciyi shodo zahistu i karantinu roslin u 2014 roci. (2014). Shkidniki ta Hvorobi Ripaku, pp:62-66 (in Ukrainian).
Prognoz fitosanitarnogo stanu agrocenoziv, rozpovsyudzhennya karantinnih organizmiv na teritoriyi Harkivskoyi oblasti ta rekomendaciyi shodo zahistu i karantinu roslin u 2015 roci (2015). Shkidniki ta Hvorobi Ripaku, p:63-67 (in Ukrainian).
Puchkov, V.G. (1956b). Yajca i lichinki nastoyashih poluzhestkokrylyh-vreditelej selskohozyajstvennyh kultur. Tr Vsesoyuz Entomology Ob-va., pp:218-342 (in Russian).
Puchkov, V.G. (1956a). Osnovnye troficheskie gruppy rastitelnoyadnyh poluzhestkokrylyh nasekomyh i izmenenie haraktera ih pitaniya v processe razvitiya. Zoology Zhurn, pp:32-44 (in Russian).
Puchkov, V.G. (1961). Fauna Ukrayini Shitniki, Kyiv: Vid-vo AN URSR (in Ukrainian).
Puchkova, L.V., Puchkov, V.G. (1958). Deyaki osoblivosti morfologiyi abdominalnih pahuchih zaloz shitnikiv (Pentatomoidea, Hemiptera-Heteroptera). DAN URSR, 1:100-104 (in Ukrainian).
Reuter, O.M. (1907). Sur quelques varietes pretendues des genres Palomena Muls. et Rey. Bulletin Social Entomology Fran, pp:209-216.
Rosenkranz, W. (1939). Die symbiose der pentatomiden (Hemiptera-Heterop tera). Ztschr Morph Oekol Tiere, 36:279-309.
Roshko, G.M. (1953). K izucheniyu shitnikov Zakarpatya. Nauch zap Uzhgor Gos un-ta., pp:67-78 (in Russian).
Roshko, G.M. (1955). K izucheniyu nastoyashih poluzhestkokrylyh Zakarpatya. Nauchn Zap Uzhgor Gos un-ta, pp:93-104 (in Russian).
Rusanova, V.I. (1926). K voprosu o biologii i okraske nekotoryh klopov iz roda Eurydema. Zash Rast ot Vreditelej, 3:378-383 (in Russian).
Saharov, N.L. (1930). Borba s vrednymi nasekomymi gorchicy, podsolnechnika, lna i drugih maslichnyh kultur. Saratov: Gos. izd-vo, Nizhne-volzh. kraevoe otd. Saratov (in Russian).
Saharov, N.L. (1934). Vrediteli gorchicy i borba s nimi. Saratov, SarkrajGIZ, pp:90-109 (in Russian).
Satubaldin, K.K. (2004). Fitosanitarnaya rol rapsa. Zashita i karantin rastenij, 9:113 (in Russian).
Schorr, H. (1957). Zur Verhaltungsbiologie und. Symbiose von Brachypelta aterrima Forst. (Cydnidae, Heteroptera). Zeit Morphol u. Okol. Tiere, 45:561-602.
Sekun, M.P. (2008). Tehnologiya viroshuvannya i zahistu ripaku. Kiyiv, Globus-Print (in Ukrainian).
Shapiro, I.D. (1951b). Rol pitayushih rastenij v biologii krestocvetnyh klopov roda Eurydema Lar. Entomol Obzor, 31:361-373 (in Russian).
Shapiro, I.D. (1951a). Rasprostranenie glavnejshih vidov krestocvetnyh klopov roda Eurydema Lar., ih vred i mery borby s nimi. Sb. rabot In-ta prikl. zool. i fitopat., 1:3-13 (in Russian).
Shegolev, V.N., Znamenskij, A.V., Bej-Bienko, G.Ya. (1937). Nasekomye, vredyashie polevym kulturam. Moscow (in Russian).
Shogolev, V.N., Strukova, M. (1932). Shkidniki olijnih kultur. Kharkiv-Kyiv, Derzhsilgospvidav (in Ukrainian).
Shogolev, V.N. (1923). Shkidniki silskogospodarskih roslin (v populyarnomu viglyadi). Kharkiv, Shlyah Osviti (in Ukrainian).
Shrejner, Ya.F. (1915) Nasekomye, vredyashie gorchice v Astrahanskoj gubernii. Zash Rast ot Vredit, 3:42-45 (in Russian).
Shtejnberg, P.N. (1907). Vrednyya nasekomyya i ispytannye sposoby borby s nimi. Saint-Petersburg, Knigoizd-vo P.P. Sojkina (in Russian).
Sitnik, I.D. (2008). Tehnologiya viroshuvannya ozimogo ta yarogo ripaka. Posibnik Hliboroba, pp:77-90 (in Ukrainian).
Stankevich, S. (2015). Shkidniki hrestocvitih. The Ukrainian Farmer, 5:74-75 (in Ukrainian).
Stankevich, S.V., Vilna, V.V. (2012b). Zalezhnist laboratornoyi shozhosti nasinnya yarogo ripaku vid peredposivnogo obrobitku insektofungicidnimi sumishami. Introdukciya, selekciya ta zahist roslin. Proceedings International Science Conference, p:169 (in Ukrainian).
Stankevich, S.V., Kava, L.P. (2013). Shkidniki ripakiv ozimogo i yarogo u Shidnomu ta Centralnomu Lisostepu Ukrayini. Visn. Hark. nac. agrar. un-tu im. V.V. Dokuchayeva. Seriya "Fitopatologiya ta entomologiya", 10:163-168 (in Ukrainian).
Stankevich, S.V., Vilna, V.V. (2012a). Vrediteli generativnyh organov yarovogo rapsa i gorchicy v Vostochnoj Lesostepi Ukrainy. Proceedings International Science Conference. “Strukturno-funkcionalnye izmeneniya v populyaciyah i soobshestvah na territoriyah s raznym urovnem antropogennoj nagruzki”, Belgorod, ID Belgorod, pp:207-208 (in Ukrainian).
Stankevich, S.V., Vilna, V.V. (2012c). Shkidniki olijno-kapustyanogo agrocenozu v umovah NNVC “Doslidne pole” HNAU im. V.V. Dokuchayeva Ekologizaciya stalogo rozvitku agrosferi i noosferna perspektiva informacijnogo suspilstva. Proceedings International Science Conference (in Ukrainian).
Stankevich, S.V., Vilna, V.V. (2012d). Vidovij sklad kompleksu hrestocvitih klopiv v umovah Harkivskogo rajonu. Dinamika bioriznomanittya 2012: zb. nauk. prac. Lugansk, LNU im. T.G. Shevchenka (in Ukrainian).
Stankevich, S.V., Vilna, V.V. (2013). Vpliv poshkodzhennya nasinnya ripaku yarogo shkidnikami z grizuchim ta kolyuche-sisnim rotovim aparatom na jogo laboratornu shozhist. Ekologizaciya stalogo rozvitku i noosferna perspektiva informacijnogo suspilstva. Proceedings International Science Conference (in Ukrainian).
Stankevich, S.V., Vilna, V.V. (2014a). Yakisni pokazniki nasinnya ripaku yarogo zalezhno vid protruyuvannya ta poshkodzhennya lichinkami ripakovogo kvitkoyida. Bulletin V.V. Dokuchayev Kharkiv National Agrarian University, 8:114-120 (in Ukrainian).
Stankevich, S.V., Vilna, V.V. (2014b). Shkidniki generativnih organiv ripaku yarogo j girchici u Shidnomu Lisostepu Ukrayini. Entomologichni chitannya pam’yati vidatnogo vchenogo-entomologa profesora M.P. Dyadechka. Proceedings International Science Conference, pp:43-44 (in Ukrainian).
Stankevich, S.V. (2013). Vpliv poshkodzhennya nasinnya ripaku yarogo lichinkami ripakovogo kvitkoyida na kilkisni ta yakisni pokazniki vrozhayu. Ekologizaciya stalogo rozvitku agrosferi i noosferna perspektiva informacijnogo suspilstva. Proceedings International Science Conference, pp:75 (in Ukrainian).
Stankevich, S.V., Teslina, V.V., Ozhga, I.I. (2010). Vnesennya dobriv yak neobhidnij element integrovanogo zahistu olijnih kapustyanih kultur. Proceedings International Science Conference, “Ekologizaciya stalogo rozvitku agrosferi i noosferna perspektiva informacijnogo suspilstva”, pp:102-103 (in Ukrainian).
Stankevych, S., Zabrodina, I., Filatov, M. (2021). Flea beetles (Phyllotreta spp.): species composition, range, bioecological features, harmfulness and protection measures. Review. Ukrainian Journal of Ecology, 11:154-168.
Stankevych, S.V., Biletskyj, Ye.M., Zabrodina, I.V. (2020). Prognostication algorithms and predictability ranges of mass reproduction of harmful insects according to the method of nonliner dynamics. Ukrainian Journal of Ecology, 10:37-42.
Stankevych, S.V., Biletskyj, Ye.M., Zabrodina, I.V. (2020). Cycle populations dynamics of harmful insects. Ukrainian Journal of Ecology, 10:147-161.
Stankevych, S.V., Biletskyj, Ye.M., Zabrodina, I.V. (2020). Prognostication in plant protection. Review of the past, present and future of nonliner dynamics method. Ukrainian Journal of Ecology, 10:225-234.
Stankevych, S.V., Gulyaeva, I.I., Hornovska, S.V. (2021). Rape pollen beetle: range, bioecological features, harmfulness and protection measures. Review. Ukrainian Journal of Ecology, 11:145-153.
Stankevych, S.V., Vasylieva, Yu.V., Golovan, L.V. (2019). Chronicle of insect pests massive reproduction. Ukrainian Journal of Ecology, 9:262-274.
Stankevych, S.V., Vilna, V.V., Zabrodina, I.V. (2021). Efficiency of chemical protection of spring rape and mustard from cruciferous bugs. Ukrainian Journal of Ecology, 11:52-59.
Stankevych, S.V., Vilna, V.V., Zabrodina, I.V. (2021). Harmfulness of cruciferous bugs. Ukrainian Journal of Ecology, 11:417-428.
Stankevych, S.V., Yevtushenko, M.D., Vilna, V.V. (2019). Integrated pest management of flea beetles (Phyllotreta spp.) in spring oilseed rape (Brassica napus L.). Ukrainian Journal of Ecology, 9:198-207.
Stankevych, S.V., Yevtushenko, M.D., Vilna, V.V. (2019). Efficiency of chemical protection of spring rape and mustard from rape blossom beetle. Ukrainian Journal of Ecology, 9:584-598.
Stankevych, S.V., Yevtushenko, M.D., Zabrodina, I.V. (2020). Pests of oil producing cabbage crops in the eastern forest-steppe of Ukraine. Ukrainian Journal of Ecology, 10:223-232.
Stankevych, S.V., Yevtushenko, M.D., Zabrodina, І.V. (2019). V.V. Dokuchaiev Scientific school of Kharkiv National Agrarian University and development agricultural entomology in XIX-XXI centuries. Ukrainian Journal of Ecology, 9:170-178.
Stark, V.H. (1928). Hemiptera-heteroptera bryanskoj oblasti. Bryanskij kraj: sbornik, 2:83-92 (in Russian).
Stobiecki, St.A. (1915). Wykaz pluskwiakow (Rhynchota) zebranych w Galicji Zachodniej i svodcowej. Spraw Korn Fizjogr Ak Um Krakow, 49:126-219.
Strukova, S. (1999). Zahist ripaku vid shkidlivih komah i hvorob. Novini Zahistu Roslin, 9:24-26 (in Ukrainian).
Suhorukov, N.I. (1947). Novye preparaty dlya borby s vreditelyami ovoshnyh kultur. Sad i Ogorod, 11:26-30 (in Russian).
Suhorukov, N.I. (1953). Kompleksnye meropriyatiya po borbe s krestocvetnymi klopami na kapuste. Dostizh Nauch Uchr Krasnodarskogo Kraya: sbornik (in Russian).
Teslina, V.V., Stankevich, S.V. (2010). Shkidniki olijno-kapustyanogo agrocenozu v umovah doslidnogo polya institutu roslinnictva im. V.Ya. Yuryeva. Ekologizaciya stalogo rozvitku agrosferi i noosferna perspektiva informacijnogo suspilstva. Proceedings International Science Conference, Kharkiv, pp:107-108 (in Ukrainian).
Teyrovsky, V. (1949). Klasifikace nesnadnych pripadri u Eurydema oieraceum L. Ent. Listy, 12:109-113.
Velichko, V.V. (1951). Belaya gorchica v nechernozemnoj polose. Moscow: Selhoziz, (in Russian).
Velichkovskij, V.A. (1913). Ocherki fauny Valujskogo uezda Voronezhskoj gubernii, 9:16 (in Russian).
Vilna, V.V., Bondar, N.V. (2013). Suhorebernik lozeliyiv-rezervator shkidnikiv olijno-kapustyanih kultur. Proceedings International Science Conference, “Ekologizaciya stalogo rozvitku i noosferna perspektiva informacijnogo suspilstva”, p:22 (in Ukrainian).
Vilna, V.V., Stankevich, S.V. (2012). Vidovij sklad kompleksu hrestocvitih klopiv v umovah NNVC “Doslidne pole” HNAU im. V.V. Dokuchayeva. Zahist roslin, Kharkiv, V.V. Dokuchayev Kharkiv National Agrarian University, pp:26-27 (in Ukrainian).
Vilna, V.V., Stankevich, S.V. (2013). Hrestocviti klopi ta obmezhennya yih shkidlivosti u NNVC “Doslidne pole” HNAU im. Bulletin V.V. Dokuchayev Kharkiv National Agrarian University. Seriya "Fitopatologiya ta entomologiya", 10:64-70 (in Ukrainian).
Vilna, V.V., Stankevich, S.V. (2014). Hrestocviti klopi ta ripakovij kvitkoyid-osnovni shkidniki generativnih organiv olijnih kapustyanih kultur u Shidnomu Lisostepu Ukrayini. News of Kharkiv Enthomology Science, 1:5-11 (in Ukrainian).
Vilna, V.V. (2013a). Vpliv poshkodzhennya hrestocvitimi klopami nasinnya kapustyanih kultur na jogo laboratornu shozhist. Proceedings of International Science Conference, “Dosyagnennya i perspektivi entomologichnih doslidzhen”, pp:37-38 (in Ukrainian).
Vilna, V.V. (2013b). Dinamika chiselnosti klopiv rodu Eurydema (Hemiptera: Pentatomydae) na posivah kapustyanih kultur u NNVC “Doslidne pole” HNAU im. V.V. Dokuchayeva. News of Kharkiv Enthomology Social, 2:63-66 (in Ukrainian).
Vilna, V.V. (2013c). Osoblivosti rozvitku hrestocvitih klopiv v 2013 na yarih kapustyanih i visadkah kapusti v NNVC “Doslidne pole” HNAU im. V.V. Dokuchayeva. Proceedings of International Science Conference, “Zahist roslin u HHI stolitti: problemi ta perspektivi rozvitku” pp:28 (in Ukrainian).
Vilna, V.V. (2013d). Dinamika chiselnosti klopiv iz rodu Eurydema na posivah kapustyanih kultur na doslidnomu poli HNAU im. V.V. Dokuchayeva u 2012. Proceedings of International Science Conference, pp:81-82 (in Ukrainian).
Vilna, V.V. (2014a). Shilnist hrestocvitih klopiv u miscyah zimivli v NNVC "Doslidne pole" HNAU im. V.V. Dokuchayeva. Proceedings of International Science Conference, 2:26-28 (in Ukrainian).
Vilna, V.V. (2014b). Vliyanie povrezhdennosti krestocvetnymi klopami kapustnyh kultur na urozhaj semyan. Proceedings of International Science Conference, “Bioraznoobrazie i ustojchivost zhivih system”, pp:123-124 (in Russian).
Vilna, V.V. (2015). Vpliv poshkodzhennya hrestocvitimi klopami nasinnya ripaku yarogo sortu Otaman na yakisni i kilkisni pokazniki (2012-2014 rr.). Fundamentalni ta prikladni doslidzhennya v zoologiyi. Proceedings of International Science Conference, pp:35-37 (in Ukrainian).
Vilna, V.V., Gubaryeva, N.O., Vasilenko, K.P. (2014). Struktura yarih olijnih kapustyanih roslin ta yih poshkodzhenist hrestocvitimi klopami v NNVC “Doslidne pole” HNAU im. V.V. Dokuchayeva u 2014 roci. Proceedings of International Science Conference, “Ekologizaciya stalogo rozvitku informacijnogo suspilstva”, pp:34-36 (in Ukrainian).
Vilna, V.V., Yevtushenko, N.D., Stankevich, S.V. (2015). Rasteniya-rezervatory hrestocvetnyh klopov. Zemledelie i zashita rastenij: nauch.-prakt Zhur., 1:43-45 (in Russian).
Vodolagin, V.D. (1936). Polosatyj klop Graphosoma italicum Mull kak vreditel anisa, koriandra i tmina. Efirno-maslichnye kultury: sbornik, pp:21-24 (in Russian).
Volovik, A.S. (1953). Effektivnost primeneniya smesej DDT i GHCG v borbe s vreditelyami kapusty. Sb. rabot nauch. stud. ob-va Leningr. s.-h. in-ta, vyp. 1:62-56 (in Russian).
Vovk, I.A. (1920). Gorodni shkidniki. Harkiv, Radyanskij selyanin (in Ukrainian).
Yakovlev, V.E. (1874). Materialy dlya entomologicheskoj fauny Evropejskoj Rossii. T. I-III, Tr. Russk Entomology ob-va., 7:7-43 (in Russian).
Yevtushenko, M.D., Vilna, V.V., Stankevich, S.V. (2016). Hrestocviti klopi na ripaku yaromu j girchici u Shidnomu Lisostepu Ukrayini, Harkiv, FOP Brovin O.V. (in Ukrainian).
Yevtushenko, M.D., Stankevich, S.V. (2012). Roslini-rezervatori osnovnih shkidnikiv olijnih kapustyanih kultur. Proceedings of International Science Conference, Dokuchayev Kharkiv National Agrarian University, pp:150-152 (in Ukrainian).
Yevtushenko, M.D., Vilna, V.V. (2014). Vidovij sklad sisnih shkidnikiv ripaku yarogo i girchici ta osoblivosti biologiyi hrestocvitih klopiv. Bulletin V.V. Dokuchayev Kharkiv National Agrarian University. Seriya “Fitopatologiya ta entomologiya”, 1:70-80 (in Ukrainian).
Yevtushenko, M.D., Fedorenko, N.V., Stankevich, S.V. (2008). Vidovij sklad ta dinamika chiselnosti osnovnih shkidnikiv olijno-kapustyanih kultur v Harkivskomu rajoni. Bulletin V.V. Dokuchayev Kharkiv National Agrarian University. Seriya “Entomologiya ta fitopatologiya”, 8:47-54 (in Ukrainian).
Yevtushenko, M.D., Fedorenko, N.V., Stankevich, S.V. (2009). Efektivnist insekticidiv pri zahisti yarogo ripaku vid blishok (Phylotretta Spp.) ta klopiv (Eurydema Spp.) do cvitinnya. Bulletin V.V. Dokuchayev Kharkiv National Agrarian University. Seriya “Entomologiya ta fitopatologiya”, 8:39-43 (in Ukrainian).
Yevtushenko, M.D., Stankevich, S.V., Vilna, V.V. (2012). Najbilsh nebezpechni shkidniki ripaku j girchici na doslidnih polyah Institutu roslinnictva im. V. Ya. Yur’yeva u 2012 roci. Zahist roslin u HHI st. Problemi ta perspektivi rozvitku. Proceedings of International Science Conference, pp:34-35 (in Ukrainian).
Yevtushenko, M.D., Stankevich, S.V., Vilna, V.V. (2014). Hrestocviti blishki, ripakovij kvitkoyid na ripaku yaromu j girchici u Shidnomu Lisostepu Ukrayiny. V.V. Dokuchayev Kharkiv National Agrarian University. Kharkiv, Majdan, p:170 (in Ukrainian).
Yevtushenko, N.D., Vilna, V.V., Stankevich, S.V. (2015). Kachestvennye pokazateli semyan rapsa yarovogo sorta Otaman v zavisimosti ot protravlivaniya i povrezhdeniya krestocvetnymi klopami. Nauchnye perspektivy HHI veka. Dostizheniya i perspektivy novogo stoletiya, 3:59-62 (in Russian).
Zhuravskij, V.S. (2012). Shkidniki ripaku yarogo ta zasobi regulyuvannya yih chiselnosti v Lisostepu Ukrayini. Thesis of Doctoral Dissertation. Kyiv (in Russian).
Ziarkiewicz, T. (1953). Eurydema oleracea L. (Hemiptera-Hetergptera, Pentatomidae). Annual Universal MCS, 8:165-191.
Author Info
S. Stankevych1*, I. Zabrodina1, D. Yushchuk1, M. Dolya3, H. Balan2, R. Yakovlev3, H. Kosylovych4, Yu. Holiachuk4, N. Zakharchuk5, T. Galagan6, L. Nemerytska7, I. Zhuravska7, O. Romanov1, T. Romanova1, O. Bragin1, O. Hudym1 and I. Hordiienko12Odessa State Agrarian University, 13 Panteleimonivska St, Odessa, 65012, Ukraine
3National University of Life and Environmental Sciences of Ukraine, Kyiv, 03041, Ukraine
4Lviv National Agrarian University, 1Volodymyra Velykogo St, Dublyany, Zhovkva district, Lviv region, 80381, Ukraine
5Institute for Potato Research NAAS, 22 Chkalova St, Nemishayeve township, Borodyansky district, Kyiv region, 07853, Ukraine
6Institute of plant protection of NAAS, 33 Vasylkivska St, 03022, Kiev, Ukraine
7Zhytomyr Agricultural College, Zhytomyr, 10031, Ukraine
Citation: Stankevych, S., Zabrodina, I., Yushchuk, D., Dolya, M., Balan, H., Yakovlev, R., Kosylovych, H., Holiachuk, Yu., Zakharchuk, N., Galagan, T., Nemerytska, L.., Zhuravska, I., Romanov, O., Romanova, T., Bragin, O., Hudym, O., Hordiienko, I. (2021). Eurydema bugs: review of distribution, ecology, harmfulness, and control. Ukrainian Journal of Ecology 11 (9), 131-149.
Received: 02-Nov-2021 Accepted: 30-Nov-2021 Published: 07-Dec-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
