Review - (2021) Volume 0, Issue 0
Flea beetles (Phyllotreta spp.): Species composition, range, bioecological features, harmfulness and protection measures: Review
S. Stankevych1*, I.V. Zabrodina1, M. Filatov1, L. Sirous1, D. Yushchuk1, V. Melenti1, K. Novosad1, L. Kava2, H. Kosylovych3, Yu. Holiachuk3, I. Derevyanko1, K. Katerynchuk4, I. Kovalenko4, O. Koval4 and S. Kyrenko5Abstract
In the course of the literature critical analysis the authors paid special attention to the morphological, biological and ecological features of the flea beetles , both in Ukraine and abroad; the authors came to the conclusion that despite the considerable number of literary sources devoted to the flea beetles, theirs is still a number of its biological and ecological features which are in close connection with the protection measures for controlling it and these measures have not yet been completely clarified. The data obtained by the entomologists from different countries regarding the harmfulness of the flea beetles and its economic importance are quite controversial and also need experimental confirmation.
Keywords
Flea beetles, morphology, biology, ecology, harmfulness, economic threshold of harmfulness, integrated protection.
Introduction
Phyllotreta Steph. is one of the most numerous genera of flea beetles in the subfamily Galerucinae and comprises, according to V. Putele’s data (Putele, 1970), more than 160 species, and according to foreign scientists’ reports (Konstantinov, 1996; Lee et al., 2011), this genus comprises more than 250 species of flea beetles. V.B. Kostromitin (Kostromitin, 1980) noted that on the territory of the former USSR over 30 species were found and V.F. Paliy (Palij & Avanesova, 1975) mentioned about 50. N.N. Plavilshikov (Plavilshikov, 1994) wrote that on the territory of the European part of the former USSR there were 21 species and M.Ye. Sergeev (Sergeev, 2007) published data on 22 species. S.V. Dedyuhin (Dedyuhin, Nikitskij & Semyonov, 2005) reported that 13 species lived in Udmurtia and 12 species-in the Lipetsk region of Russia (Curikov, 2009).
Representatives of the genus damage plants of such families as Gramineae, Brassicaceae, Malvaceae, Asteraceae, Chenopodioideae, Moraceae, Leguminosae and Polygonaceae (Palij & Avanesova, 1975).
Flea beetles, which more or less damage Brassicaceae crops, include 19 species (Kostromitin, 1980) or, as V.N. Shyogolev reported (Shyogolev, Znamenskij & Bej-Bienko, 1937),-11 species. According to N.A. Filippov data (Filippov, 1978), 12 species were observed in Moldova, but G.I. Konchukovskaya (Konchukovskaya, 1978) had only data on 10 species. Yu.N. Bezdelnyj (Bezdelnyj, 1984) particularized 9 pest speciess in the Altai Territory. According to N.L. Saharov data (Saharov, 1934) mustard was damaged by 9 species in the Saratov region. There were 13 species in Tatarstan (Kosov, Rameev & Lopaeva, 1952). V.P. Razumov (Razumov, 1971) recited 7 species of flea beetles damaging Brassicaceae crops in the Gorky region of Russia; Ye.V. Levkovich (Levkovich & Levkovich, 2006)-6 species in the Penza region; and A.P. Smirnov (Smirnov, 2009) 5 species-in the Leningrad region. In the Non-Chernozem Belt of Russia, as T.I. Manaenkova described (Manaenkova, 1991), the dominant species was the small striped flea beetle (89.8%). V.G. Osipov (Osipov, 1986) pointed out that in Belarus, Brassicaceae crops were damaged by 6 species of flea beetles. Six species were also found to be pests in the Leningrad region. Of them, the predominant species was the turnip flea beetle (Guseva & Koval, 2007). According to K.A. Gorbatko data (Gorbatko, 2010), 4 species were widespread in the Central Ciscaucasia, of which the cabbage flea beetle was predominant (40% in the total population). In Ukraine, Brassicaceae crops are damaged by 6 species of flea beetles belonging to the genus Phyllotreta Steph. (Minkevich, & Borisovskij, 1949; Gerasimov & Osnickaya, 1961). In Taiwan, (Chen, Ko & Lee, 1990) the predominant species on Brassicaceae crops is the striped flea beetle. The striped flea beetle is the most common species of Phyllotreta spp. in the world (Zhao et al., 2008) and it is the most dangerous pest of Brassicaceae plants in Canada (Soroka & Elliot, 2011). In Turkey, the predominant species is the small striped flea beetle.
V.P. Vasilev (Vasilev, V.P. et al., 1988) wrote that the cabbage flea beetle and the small striped flea beetle predominated in the forest-steppe of Ukraine, accounting for 60-90% of the total Phyllotreta spp. number. O.M. Lapa (Lapa et al., 2006) pointed out that the cabbage flea beetle predominated in the South of Ukraine, while the small striped flea beetle, the striped flea beetle and the turnip flea beetle-in the North. M.P. Sekun (Sekun et al., 2008) believed that the cabbage flea beetle and the small striped flea beetle predominated in the woodlands and forest-steppe, while the turnip flea beetle-in the South of Ukraine.
Cabbage flea beetle (Phyllotreta atra F. and Ph. atra var. cruciferae Goeze.)
It lives in the European part of Russia, the Caucasus, Central Asia, Kazakhstan, Siberia, and Primorye, being one of the most widespread pests in the steppe zone from the Baikal region to the Balkans. Outside the former Soviet Union, it can be found in Western Europe, Asia Minor, Central Asia, and northeastern Africa. Variation cruciferae predominates in more northern regions. In the early 1920s, it was brought to the west coast of North America, then quickly spread throughout the continent and became the predominant species, inflicting significant damage to Brassicaceae crops (Palij & Avanesova, 1975; Kostromitin, 1980) (Fig. 1).
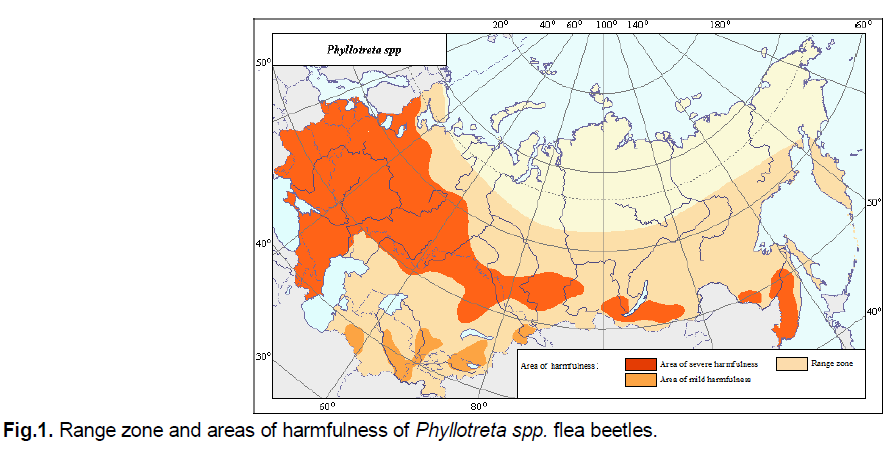
Figure 1: Range zone and areas of harmfulness of Phyllotreta spp. flea beetles.
Small striped flea beetle (Ph. undulata Kutsch.)
Its area (except Ukraine) is the European part of the former Soviet Union, the Caucasus, Siberia, Primorye, Central Asia, Kazakhstan. It is one of the most dangerous pests of Brassicaceae crops, annually damaging plants in Karelia, the Leningrad, Moscow, Gorky, Kalinin, Pskov, Arkhangelsk, Perm, Kirov, Vologda regions, Western and Eastern Siberia, the Middle Urals, Yakutia and the Far East. Significant damage was observed in Belarus, the Smolensk, Kaluga, Tula, and Ryazan regions, Chuvashia and Tatarstan. Outside the former Soviet Union, it ranges in Europe, with the exception of Greece, Asia Minor, and Algeria. Ph. undulata was brought to the United States (Samedov, 1963; Palij & Avanesova, 1975; Kostromitin, 1980).
Turnip flea beetle (Ph. nigripes F.)
On the territory of the former USSR, it is very widely spread: forest-steppe and steppe of the European part of Russia, the Caucasus, Central Asia, Kazakhstan, Western Siberia. Ph. nigripes usually damages plants together with Ph. atra and Ph. undulata. Mass reproduction occurs in years with warm springs and normal precipitation in the Southern Urals, southern Western Siberia, sometimes in the Kursk, Voronezh and Penza regions. Outside the former USSR, it is known in Europe, Asia Minor, and northwestern Africa (Samedov, 1963; Palij & Avanesova, 1975; Kostromitin, 1980).
Turnip flea beetle or yellow-striped flea beetle (Ph. nemorum L.)
Within the former USSR, in addition to Ukraine, the range of this species is large and covers the entire European part, the Caucasus, Eastern and Western Siberia, the Far East and the republics of Central Asia. Mass reproduction occurs in humid regions of the Baltic countries, Belarus, the Leningrad, Smolensk, Kaliningrad, Vologda, Pskov, and Moscow regions, the Caucasus. It can be found in Karakalpakstan and Uzbekistan. In wet years, it also appears in arid areas. Outside the former USSR, it is widespread in Western Europe and Asia Minor (Samedov, 1963; Palij & Avanesova, 1975; Kostromitin, 1980).
Striped flea beetle (Ph. striolata Fabr.Ph. vittata Fabr.)
On the territory of the former USSR, it inhabits the entire European part, the Caucasus, Kazakhstan, Siberia, Primorsky Kray. The striped flea beetle number is growing in Siberia and the Far East. Outside the former USSR, the range of Ph. striolata is very large: it ranges in Europe, Japan, China, Mongolia, Thailand, on the island of Sumatra, South Africa, USA. In North America, it is a serious pest of Brassicaceae crops (Samedov, 1963; Palij & Avanesova, 1975; Kostromitin, 1980).
Horseradish flea beetle (Ph. armoraciae Koch.)
In addition to Ukraine, it lives wherever horseradish (Ph. armoraciae feeds only on horseradish) grows, which is harmed by both imagoes and larvae. The range of the species covers the southeastern European part of Russia, the Caucasus, Central Asia, Kazakhstan, Central, Eastern and Western Siberia, the Leningrad, Novgorod, Moscow, Ryazan, and Ulyanovsk regions, Central and Southern Urals, Bashkortostan, Tatarstan, Mordovia. It lives in the Baltic countries and Uzbekistan. Outside the former USSR, it ranges in Europe (except the Iberian Peninsula), Canada, and the United States (Samedov, 1963; Palij & Avanesova, 1975; Kostromitin, 1980).
Several European researchers, Hoffmann (Hoffman & Schmutterer, 1983), Yonen (Johnen, 2006; Johnen & Klingenhagen, 2006), Knoll (Knoll, 1997), and Volker (Volker, 2003) noted that it was Phyllotreta spp. flea beetles that were most harmful species on rapeseed in Belgium, Bulgaria, Germany, Poland, Slovakia, and France.
In the Russian Empire, Phyllotreta spp. flea beetles significantly damaged to cabbage and mustard plantations, so as early as in the late 1800s, such scientists as K. Lindeman (Lindeman, 1866), K.P. Bramson (Bramson, 1881), F. Keppen (Keppen, 1882), K.A.
Purievich (Purievich, 1893) and P.M. Stejnberg (Shtejnberg, 1907) conducted thorough research into their biological features and effective measures to control them. In 1908, Phyllotreta spp. flea beetles were included in the list of the most harmful insects for agriculture.
Information on the harmfulness of Phyllotreta spp. flea beetles in the eastern forest-steppe of Ukraine was published in the early 1900s, where pests of the Kupyansk, Bohodukhiv and Valkiv Uyezds were described. It was noted that 3 species of Phyllotreta spp. were harmful: Ph. atra F., Ph. undulata Kutsch. and Ph. nemorum L.
We revealed that all 6 species of flea beetles that were common in Ukraine could be found in the eastern forest-steppe of Ukraine (Stankevych & Fedorenko, 2009; Yevtushenko, Stankevych & Fedorenko, 2009; Krasilovec et al., 2011; Krasilovec, Kuzmenko, Litvinov & Stankevich, 2011; Stankevych, 2011c; Stankevych, 2012d; Stankevych, 2012e).
Materials and Methods
The authors analyzed 196 literary and electronic sources from the late 19th to the 21st century. During the analysis, special attention was paid to the morphological, biological and ecological features flea beetles in Ukraine and abroad. The data on the harmfulness of the flea beetles and its economic significance are especially analyzed. In the course of the analysis special attention was paid to the methods and ways of controlling the rape pollen beetle in Ukraine and abroad. The protective measures were considered in such directions as agro-technical, physic and mechanical, chemical, biological, biotechnical and selective and genetic ones. Each of them is noteworthy and has both a number of disadvantages and indisputable advantages in comparison with other methods.
Results and Discussion
Taxonomic status and morphological features of Phyllotreta spp. flea beetles
V.B. Kostromitin (Kostromitin, 1980) and N.N. Plavilshikov (Plavilshikov, 1994) specified the taxonomic status of the genus Phyllotreta is follows: Class Insects-Insecta Leach, 1815; Subclass Winged or Higher Insects-Pterygota Gegenbaur, 1878; Infraclass Neopterans-Neoptera Martynov, 1923; Division Distinct-Stage Insects-Holometabola; Superorder Coleopteroids-Coleopteroidea; Order Coleopterans-Coleoptera Linnaeus, 1758; Suborder Omnivorous Beetles-Polyphaga Emery, 1886; Family Leaf Beetles-Chrysomelidae Spribala, 1802 Aphthonini; Genus Phyllotreta Stephens, 1836.
Representatives of the genus Phyllotreta have an elongated and mostly flattened body; the body is unicolourous (black, blue, greenish, metalescent or black with a yellow pattern on elytrons). The head with indistinct frontal tubercules or without them; the frontal carina is flat or sharp, narrow. The labrum is square with a notch on the anterior edge; the mandible is five-toothed; the antennae are 11-segmented. The pronotum in most species is square, narrower at the elytron base; the clypeus is small, semi-oval; the shoulder humps are mostly convex. Almost all species have well-developed hind wings. The body length varies 1.3 to 3.5 mm (Troickij & Shegolev, 1934; Shyogolev, 1960).
Eggs of Phyllotreta spp. flea beetles are light yellow, semi-translucent, elongated-oval, 0.34-0.4 mm long and 0.1-0.2 mm wide (Narzikulov, 1968). However, according to N.N. Bogdanov-Kat’kov data (Bogdanov-Kat’kov, 1920), the egg size is 0.6-0.9 mm.
Larvae of most species are off-white, yellow or yellowish. On the surface of the segments, there are smooth, shiny sclerotized plates, arranged in a certain order. There is one hair on each plate. Some plates merge together, and then the number of hairs increases according to the number of merged plates. The head and the last segment are light yellow. The tergite of the last abdominal segment is without cancellated structure, has a rounded posterior edge or one upfracted short chitin hook (Gilyarov, 1964)
Pupae of all species are free, yellowish, 2-3 mm (up to 4 mm) long, always develop in the soil (Shyogolev, Znamenskij & Bej-Bienko, 1937; Palij, 1962; Shapiro, 1964).
Characteristic features of Phyllotreta spp. flea beetles that are common in Ukraine are the following:
The upper part of the body is unicolourous: black, blue or greenish.
Cabbage flea beetle: The antennae are black, except for the first three red-yellow segments. The proximal part of the legs is grayish brown (Fig. 2E). The head, pronotum and elytrons are evenly dotted. The color is black with a faint metallic tinge. The length is 1.8-3.0 mm (Gerasimov & Osnickaya, 1961; Bej-Bienko, G.Ya. (1980).
Turnip flea beetle: The antennae and legs are completely black. The upper part of the body is blue or greenish with a metallic tinge (Fig. 2F). The head and anterior back are finely dotted. The length is 2.0-2.8 mm (Palij & Avanesova, 1975).
Turnip flea beetle: There are yellow stripes on the elytrons with a rather subtle notch in the middle or almost parallel (Fig. 2A). The tibiae and legs are red or yellow (as B.A. Gerasimov (Gerasimov & Osnickaya, 1961) described). The frons and at least the frontal part of the crown are not dotted. The head and pronotum are metalescent. The main 3 segments of the antennae are yellow. It is one of the largest species: 2.5-3.5 mm (Gerasimov & Osnickaya, 1961; Lopatin, 1986).
Small striped flea beetle: The black side border on the elytrons does not expand or expands very gradually and peripherally into the yellow stripe (Fig. 2B). The legs are black and only the tibiae are sometimes slightly reddish. The frons has a transverse dotted stripe only above the tubercules; the crown is not dotted (Troickij & Shegolev, 1934). The length is 2.0-2.8 mm (Lopatin, 1986).
Striped flea beetle: On the elytrons, there is a yellow stripe with a deep outer notch in the middle and a small notch near the shoulder hump (Fig. 2D). There are sometimes 2 spots. Black stripe in the middle, with parallel edges, narrowed only at both ends. The length is 1.8-2.7 mm and the width is 1.1-1.4 mm (Gerasimov & Osnickaya, 1961; Palij & Avanesova, 1975; Lee et al., 2011).
Horseradish flea beetle: The elytrons are yellow; only the narrow border on their outside and the stripe on the neck are black (Fig. 2C). The head and pronotum are black. The top of the femora, tibiae, legs and the first 3 segments of the antennae are yellow. The length is 3.0-3.5 mm (Gerasimov & Osnickaya, 1961).
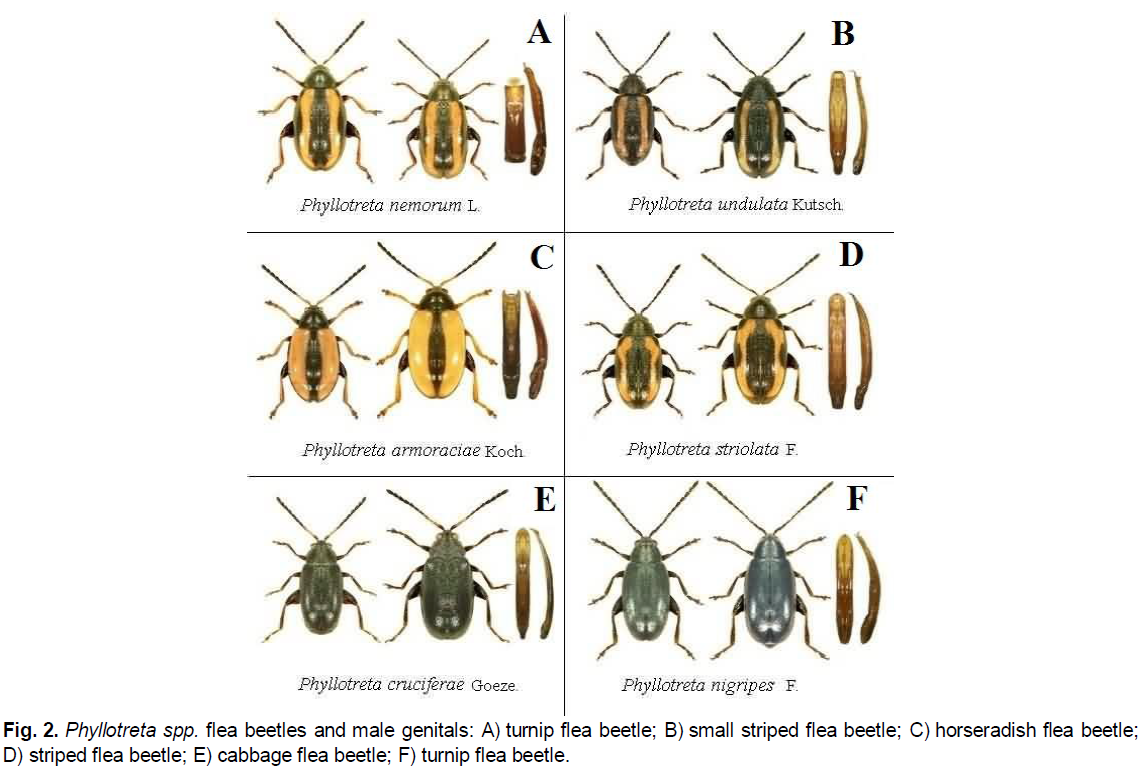
Figure 2: Phyllotreta spp. flea beetles and male genitals: A) turnip flea beetle; B) small striped flea beetle; C) horseradish flea beetle; D) striped flea beetle; E) cabbage flea beetle; F) turnip flea beetle.
Biological and ecological features of Phyllotreta spp. flea beetles
Phyllotreta spp. flea beetles have similar biological features (Laba & Sytnyk, 2006). Sexually immature imagoes overwinter in the upper layer of the soil, in cracks of greenhouse frames, under fallen leaves on forest edges and in protective forest belts (Shyogolev, Znamenskij & Bej-Bienko, 1937). However, L.I. Bud’ko (Bud’ko, Rovba, & Shaganov, 2008) published data that eggs overwinter in the soil in Belarus and larvae emerge from them in spring (at 5-6°С), which damage seedlings. Sometimes imagoes get into soil cracks between roots and imbed themselves in the soil. They rarely overwinter in meadows and fields. Imagoes normally overwinter in the upper layer of the soil, where the temperature is -4°C under the snow cover. Phyllotreta spp. flea beetles seldom overwinter far from the places of their autumn feeding and are satisfied with any cover in the immediate vicinity of the autumn habitat, and hence their spring appearance is close to those places where Brassicaceae plants grew in the previous year. In spring, beetles come out of overwintering housings and feed additionally (Dobrovolskij, 1950; Kasyanov, 2011). The appearance of beetles is closely associated with air temperature (Marchenko, 2011). First they appear in areas that are well warmed up. T.G. Yefremova (Yefremova,1970) reported that in the southern regions of Ukraine beetles appeared as early as during the third ten days of March, while in the northern and central regions-in the second ten days of April, and in Kamchatka (Semakov, 1966), for example, beetles leave overwintering housings in early June.
I.M. Zambin (Zambin, Turaev & Shumilenko, 1953) believed that flea beetles came out of overwintering housings when the average daily temperatures rose to 11°C. Different species of Phyllotreta spp. do not become active simultaneously; their activity onset depends on the spring weather: the later spring comes and the cooler the weather is, the later flea beetles appear (Skripnik & Zhuravskij, 2004). At this time, there are no cultivated plants in the fields and beetles feed on various Brassicaceae weeds, but after seeds germinate or after seedlings are planted in the soil, beetles move to them, inflicting considerable damage. Phyllotreta spp. imagoes begin to fly at an air temperature of 14-16°C (Lopatin, 1986). During the day, the intensity of flight changes significantly: it increases as the air temperature rises and decreases markedly when the cool evening comes. In the evening, imagoes are sluggish and one can easily count them. N.N. Bogdanov-Kat’kov (Bogdanov-Kat’kov, 1920) reported that the maximum activity of imagoes was observed during the day at ≥17°C. According to V.N. Shyogolev observations (Shyogolev, Znamenskij & Bej-Bienko, 1937), imagoes are most active from 10 a.m. to 1 p.m. and later from 4 p.m. to 6 p.m. Nevertheless I.S. Iskakov (Iskakov & Krasnikova, 1991) reported that flea beetles actively fed after 6 p.m. if the air temperature did not drop below 18°C. Excursions of flea beetles are sharply reduced in cloudy weather and completely stop in rainy weather. Winds can carry flea beetles hundreds and thousands of meters away. The distance of flea beetle excursions depends on wind speed. In spring, in favorable weather, the Phyllotreta spp. flea beetle numbers are stable and they are more or less evenly distributed in different landscapes, but gradually they got accumulated at certain stations. Phyllotreta spp. flea beetles migrate from one station to another throughout the growing period. Beetles move more intensively before egg laying; they migrate to Brassicaceae fields. Obviously, domestic plants are better food for flea beetles. By the end of May, tissues of wild plants become coarser; the formation of generative organs requires the outflow of macronutrients from leaves, the amounts of water and protein in leaves decreases sharply; and this is most likely to make beetles migrate to young, intensively growing, water- and protein-rich leaves of domestic plants.
In winter, imagoes spend their fat reserves. Phyllotreta spp. flea beetles start feeding immediately after spring activation. M.G. Alimbekova (Alimbekova, Kukshina & Nikitina, 1949) indicated that imagoes could survive without food for 10-12 days, but according to other researchers’ data (Kostromitin, 1980), the life span of imagoes without food was 5-6 days in a laboratory experiment.
In spring after overwintering, Phyllotreta spp. flea beetles always need extra nutrition. We observed (Stankevych, 2011c; Yevtushenko, & Stankevich, 2012) that after Phyllotreta spp. had left their leaving overwintering housings, they could be found on Sisymbrium, flixweed, charlock mustard and winter cress. At this time, the quality of food is not important, but as reproductive products ripen, additional nutrition can be only satisfied by Brassicaceae plants or by very taxonomically close Capparaceae and Resedaceae plants. Of the species of Phyllotreta spp. that are common in Ukraine, none can be classified as monophagous, as even such seemingly specialized species as the horseradish flea beetle, feed on at least several plant species. Thus, most species belong to broad or to narrow oligophages. Mustard oils or their glucosides contained in plants are believed to be active attractants for Phyllotreta spp. flea beetles and play an important role in the insect’s choice of the host plant. At various times, it was experimentally shown that even bean leaves, which are not normally consumed by Phyllotreta spp. imagoes, become edible to them after such leaves had been seasoned in a 0.5% aqueous solution of a mustard oil glucoside for 18 hours. Plants of the families Resedaceae, Chenopodiáceae, Gramíneae and Asteraceae can be suitable food for Phyllotreta spp. flea beetles. Phyllotreta spp. imagoes damage plants of the families Amarantháceae, Polygonáceae and Fabáceae if only other food is not available. For example, O.V. Gordiyenko (Gordiyenko, 2008) pointed out that Phyllotreta spp. flea beetles together with Chaetocnema spp. flea beetles were the main pests on buckwheat seedlings. Although under natural conditions flea beetles can feed on some plant species that are not their eating habits, the normal development of Phyllotreta spp. flea beetles only occurs when they feed on Brassicáceae plants. M.G. Alimbekova (Alimbekova, Kukshina & Nikitina, 1949), D.S. Shapiro (Shapiro, 1964) and V.F. Paliy (Palij, 1962) even emphasized that it was imposible for Phyllotreta spp. flea beetles to eat non-Brassicáceae plants. Feeding on Brassicáceae plants promotes the development of viable and fertile specimens of Phyllotreta spp. Eating leaves of plants of other families is not typical for Phyllotreta spp. flea beetles; it is rather random than typical, though can be sometimes very noticeable. Phyllotreta spp. flea beetles very willingly feed on many Brassicáceae crops, for which they are permanent and mass pests. Phyllotreta spp. flea beetles inflict the greatest damage to mustard, rapeseed, cabbage, turnip, and oil radish; radish, false flax, horseradish, rutabaga and white turnip are also severely damaged by Phyllotreta spp. flea beetles (Bogdanov-Kat’kov, 1920).
The period of additional feeding lasts from 5 to 60 days (Bogdanov-Kat’kov, 1920; Palij & Avanesova, 1975; Kostromitin, 1980).
After imagoes complete additional feeding and reach sexual maturity, they mate and lay eggs, mainly in fields sown with cultivated Brassicáceae plants; Ph. nemorum lay eggs on wild Brassicáceae plants (Alimbekova, Kukshina & Nikitina, 1949; Palij, 1954). Eggs are laid in the soil, and larvae that hatch from eggs feed on small roots of Brassicáceae plants, without causing significant damage (Kolesnik, 2007). A.V. Melnik (Melnik, 2007). reported that the flea beetles laid eggs in holes gnawed in roots of Brassicáceae plants. Ph. striolata females gnaw holes in the main root of a plant and lay eggs in there; larvae develop inside the roots of oil radish, radish and other Brassicáceae crops. Ph. nemorum lay eggs on the underside of leaves of Brassicáceae plants, mainly white charlock and radish. Larvae gnaw into leaves and live there until pupation. V.N. Shyogolev (Shyogolev, Znamenskij & Bej-Bienko, 1937) pointed out that Ph. undulata females also laid eggs on leaves of Brassicáceae crops and larvae developed in leaves. Females of the horseradish flea beetle lay eggs (sometimes up to 16 eggs) near the horseradish collet (Kostromitin, 1980). According to B.V. Dobrovolskij data (Dobrovolskij, 1950), females lay about 20 eggs near roots. The total productivity of females is about 40 eggs (Lopatin, 1986); according to D.N. Kobakhidze data (Kobahidze, 1957), it ranges 40 to 60 eggs. N.N. Bogdanov-Kat’kov (Bogdanov-Kat’kov, 1920) reported that flea beetles lay single eggs or egg clusters on the surface of plants. Most researchers thought that the duration of embryonic development depended on the soil temperature and lasted from 3 to 15 days (Dobrovolskij, 1950; Kobahidze, 1957; Gerasimov & Osnickaya, 1961; Shutak, 1973; Palij & Avanesova, 1975; Kostromitin, 1980).
Larvae of the horseradish flea beetle penetrate into horseradish leaf stalks, where they further develop; they can also develop in the main veins of cabbage, mustard and rapeseed leaves. Having completed feeding, Ph. nemorum and Ph. armoraciae larvae, come out of leaves and pupate in the soil, like other species. The development of larvae lasts 14-30 days (Dobrovolskij, 1950; Kobahidze, 1957; Osmolovskij, 1972; Shutak, 1973; Palij & Avanesova, 1975; Kostromitin, 1980). All species of Phyllotreta spp. flea beetles pupate exclusively in the soil at a depth of 1-12 cm (Shyogolev, Znamenskij & Bej-Bienko, 1937; Dobrovolskij, 1950). The development of pupae takes from 8 to 17 days (Bogdanov-Kat’kov, 1920; Shutak, 1973; Palij & Avanesova, 1975; Kostromitin, 1980). The whole development lasts from 27 to 50 days (Bogdanov-Kat’kov, 1920; Palij & Avanesova, 1975; Kostromitin, 1980).
At the end of July, a new generation of flea beetles emerges. Young beetles also feed on different Brassicáceae plants and, when it gets cold, they move to overwintering housings. Most researchers (Kovalchuk, 1987; Fedorenko et al., 2008) thought that Phyllotreta spp. flea beetles developed one generation throughout Ukraine, while V.D. Pyatakova (Pyatakova, 1928) and A. Podkopayev (Podkopayev, 1933) noted that Ph. atra and Ph. nemorum could develop even three generations in Ukraine. KK Fasulati (Fasulati, 1963) reported that in Transcarpathia Ph. undulata gave two generations and V.T. Melnychuk (Melnychuk, 1996) published data on two generations of Ph. undulata in the forest-steppe. D.N. Kobakhidze (Kobahidze, 1957) noted that Phyllotreta spp. flea beetles had two generations per year on the Black Sea coast. According to N.N. Bogdanov-Kat’kov (Bogdanov-Kat’kov, 1920) and L.V. Sazanova (Sazanova, 1955) data, Phyllotreta spp. flea beetles develop 2 generations in the southern regions of the former USSR. In the Magadan region (Bardysheva, 1967), Phyllotreta spp. flea beetles give 1 generation per year. A.A. Solovyova (Solovyova, 1970) reported that flea beetles gave in 3 generations Kyrgyzstan and 1 generation in the highlands (1500 m above sea level). In the Central Caucasus, as K.A. Gorbatko (Gorbatko, 2010) mentioned, there is one generation of flea beetles per year.
In summer, when a new generation of beetles appears or after harvesting early Brassicáceae crops due to lack of food, Phyllotreta spp. flea beetles migrate to wild plants, especially if they grow nearby. Of the wild Brassicáceae plants, beetles prefer nasturtium, Sisymbrium, hoary alyssum, flixweed, hoary pepperwort, colewort, Alliaria, wallflower, winter cress, white charlock, stock, pennycress, etc. Ph. undulate and Ph. atra eat leaves of the shepherd's purse and Ph. nigripes-leaves of the candytuft (Bogdanov-Kat’kov, 1920; Moric-Romanova, Berezhkov & Davydov, 1941; Zambin, Turaev & Shumilenko, 1953; Gerasimov & Osnickaya, 1961; Kostromitin, 1980). Trophic specialization determines the spread of Phyllotreta spp. flea beetles. Stable food reserves are available near human settlements, where cultivated, weed and ornamental plants belonging to the family Brassicáceae are abundant. In natural biocenoses, edible plants are poorly represented; Phyllotreta spp. flea beetles are sure to live there, but in smaller numbers. It was published (Kostromitin, 1980) that the numbers of beetles that overwintered and their offspring were always higher in fields of cultivated plants than on wild vegetation growing near the fields. An agrocenosis of Brassicáceae crops is populated faster and damaged more severely than a Brassicáceae crop grown in a mixture with other crops (Satalkina & Ancupova, 1993). Thus, the diversity of vegetation can reduce the numbers of Phyllotreta spp. flea beetles and prevent their mass reproduction.
Harmfulness of Phyllotreta spp. flea beetles
Data on the harmfulness of Phyllotreta spp. flea beetles have been available since 1841 (Bogdanov-Kat’kov, 1920). The damage from them was so significant that in some years (1841, 1851, 1867, 1888, 1911, 1913, 1915) they destroyed all planted cabbage seedlings and oilseed fields (Bogdanov-Kat’kov, 1920).
The damage caused by Phyllotreta spp. flea beetles to fields depends mainly on their numbers, migration capacity, phase of the plant development, feeding intensity, weather, etc. The feeding activity of beetles depends on weather factors. Beetles start feeding after the dew evaporates and the air temperature reaches 7-9°C. As the temperature rises, the feeding intensity increases. The optimal temperature for active feeding of beetles is within 18-25°С (Shejgerevich, 1988); its further increase to 27-29°С noticeably reduces the feeding intensity; and at 30-32°С almost all beetles leave plants. In the evening, when the air temperature drops to 11-12°С, the activity and intensity of feeding lessen and after 9 p.m. beetles probably go into the soil for the night, because early in the morning they can be detected only on the soil surface.
Training, Research and Production Center Doslidne Pole (Experimental Field) of VV Dokuchaev KhNAU (the first 10 days of June, 2019).
State Enterprise “Experimental Farm Elitne” of the Plant Production Institute named after VYa Yuriev of NAAS (2012)
In most species of Phyllotreta spp. flea beetles, imagoes do harm; larvae develop in the soil, feeding on small roots, and do not have a significant impact on the plant growth and development (Fokin, 2008; Pysarenko & Gordyeyeva, 2009). However, Ph. nemorum larvae penetrate into leaves and live there until pupation. Larvae of the horseradish flea beetle develop inside the middle veins of horseradish and cabbage leaves (Palij, 1962; Kostromitin, 1980).
Beetles appear en masse on young Brassicáceae plants, i.e. on seedlings (grown from seeds in the fields or planted from pots) (Palij, 1962). They eat cotyledons and the youngest, apical leaves, scrape the epidermis of leaves, resulting in ulcers of different diameters (Fig. 3) (Pysarenko & Gordyeyeva, 2009) and, according to N. P. Kosmodemyanskij data (Kosmodemyanskij & Kulik, 1967) skeletonize leaves of Brassicáceae plants upon mass reproduction.

Figure 3: Phyllotreta spp. flea beetles on spring rapeseed leaves.
As N.N. Bogdanov-Kat’kov (Bogdanov-Kat’kov, 1920) observed, one flea beetle gnaws out an ulcer of 2.5-3.0 mm in 10 minutes and in total one flea beetle gnaws out 14-15 mm2 of the leaf surface per day. V.D. Pyatakova (Pyatakova, 1928) pointed out that 10 flea beetles ate 430 mm2 of the leaf surface at 14.3°C 720 mm2 at 20.6°C.
Beetles of the new generation very often damage stems, flowers, pods, and fruits (Fig. 4) (Susidko & Pisarenko, 1991). Ulcers are grouped in several places and often on the edges of cabbage leaves; on rapeseed, mustard, radish, white turnip, rutabaga and radish, ulcers are scattered over the entire surface of leaves. Damaged tissues get dry, discolor and small holes are formed on leaves (Legatov, 1929). Upon severe damage, leaves turn yellow, their normal development is broken and they dry up. Plants lag behind in growth and young plants die.

Figure 4: Plants damaged by young imagoes of Phyllotreta spp. А) Damaged pod; В) Damaged stem.
Recently, however, their numbers on Brassicáceae crops have increased several-fold compared the above figures. Biotic factors (predators, parasites, pathogens) do not limit the numbers of Phyllotreta spp. flea beetles within the ETH, so insecticides are used to protect plantations against them (Osipov, 1986). D.M. Korolkov (Korolkov & Durnovo, 1926) pointed out that in hot weather, upon mass development, flea beetles were able to obliterate seedlings on the germination day. After beetles have eaten up 50% of the leaf surface of cotyledons, plants quickly lose their vitality, many of them die, and survivors give significantly reduced yields. In years of their mass reproduction, Phyllotreta spp. flea beetles completely wipe out young shoots (Zambin, Turaev & Shumilenko, 1953). Flea beetles also damage seed plants of Brassicáceae crops, eating out small (1.5-2.0 mm in diameter) ulcers on buds and pods (Troickij & Shegolev, 1934; Shyogolev, 1960). Damage to leaves of older plants delays their growth and reduces yields. Flea beetles willingly feed on flower heads, especially on wild Brassicáceae plants right after coming into blossom. One can often watch flea beetles on the leaf surface unbending parts of the flower head and gnawing at the base of the flower rachis. They often completely gnaw through rachises and flowers dry up. Oil crops are damaged to varying degrees by Phyllotreta spp. flea beetles. Brown mustard, white mustard, Chinese radish and spring rapeseed seedlings are more severely damaged, while spring turnip rape seedlings are slightly less attackable, colewort seedlings are little damaged, and false flax seedlings are almost invulnerable (Kostromitin, 1980). According to M.V. Kalyuga data (Kalyuga, 1970), different Brassicáceae crops are unequally valued food for insects that feed on them. Their nutritional value is determined by contents of nitrogen and monosaccharides.
Protection measures against Phyllotreta spp. flea beetles
Information about measures to control Phyllotreta spp. flea beetles has been available since the mid-nineteenth century. K.P. Bramson (Bramson, 1881) and F. Keppen (Keppen, 1882) recommended growing Brassicáceae plants in shady sites, because, in their opinion, they are unfavorable conditions for flea beetles. They also recommended exterminating flea beetles by gathering them with a sweep-net or with tar-anointed boards, which were placed on a small cart. The moving cart makes flea beetles jump and they got stuck to the tar. V.Ye. Iversen (Iversen, 1883) recommended carrying wooden frames with stretched tar-anointed cloths above plantations and thus catching frightened beetles. Vegetating plants were recommended to be sprinkled with ash, lime dust, or with ground bird or horse manure (Shtejnberg, 1907). This operation had to be repeated after each rain. Plants could be also watered with so-called "wormwood water" (a handful of wormwood in a bucket of water) (Iversen, 1883). It was recommended to add gypsum, guano, garlic or wood ash to wormwood water. F. Keppen (Keppen, 1882) recommended spreading horse manure between plant rows with its subsequent burning as an effective measure. He observed that flea beetles, which were frightened by acrid smoke, completely left the field one hour later. It was recommended to repeat this operation every ten days. A. Blomejer (Blomejer, 1901) published data on the effectiveness of sprinkling the perimeter of rapeseed fields with dry horse manure (the band width was 2-4 m), which prevented attacks of flea beetles on fields in spring. F. Keppen (Keppen, 1882) also recommended double seeding rates because in this case flea beetles did not kill all plants. The following F. Keppen (Keppen, 1882) and V.Ye. Iversen (Iversen, 1883) recommendations are also of interest: to sow seeds of wild Brassicáceae plants/Brassicáceae weeds near plots of domestic Brassicáceae plants, then most flea beetles fed on the former and there could be caught with sweep-nets. This can be considered as the start of using trap plants. V.Ye. Iversen (Iversen, 1883) recommended pre-sowing one-day soaking Brassicáceae seeds in garlic or sulfur mixture and sowing the seeds as early as possible, before flea beetles appear en masse. In the fields of Brassicáceae crops, it was mandatory to eradicate Brassicáceae weeds (Iversen, 1883).
A. Blomejer (Blomejer, 1901) wrote about the low efficiency of sweep-nets, tar-anointed boards and tobacco dust sprinkling and emphasized that good fertilizers and tillage, i.e. agronomic measures should be priorities.
In a review of pests of Kupyansk Uyezd (Obzor vrednyh nasekomyh Kupyanskago uezda po nablyudeniyam 1905 goda, 1906), it was recommended to use long boards with a tar-anointed cloth nailed in the upper part. The cloth bottom was left dry. Then two workers carried it across the field, touching plants with the lower edge and catching frightened flea beetles. Putting this contrivance on a cart, one could make a so-called "Gottingen cart" and significantly accelerate the catch of flea beetles.
P.N. Stejnberg (Shtejnberg, 1907) demostrated the effectiveness of sprinkling plants with Thomas slag. This by-product of cast-iron production, in addition to its negative effect on flea beetles, is a valuable phosphorus fertilizer, which is still used at present. In P.N. Stejnberg publication (Shtejnberg, 1907), copper acetoarsenite or Schweinfurt green was mentioned for the first time as an insecticide against Phyllotreta spp. flea beetles.
In the 1920s, to protect crops against Phyllotreta spp. flea beetles, it was recommended to spray plants with copper acetoarsenite, barium chlorate and lead orthoarsenate, to apply sticky catchers, to sprinkle plants with Thomas slag or with ash, and to eradicate Brassicáceae weeds (Bogdanov-Katkov, 1920; Korolkov & Durnovo, 1926).
In the 1930s, it was recommended to spray with copper acetoarsenite, barium chloride, calcium orthoarsenate, sodium orthoarsenate, or with sodium silicofluoride, to sprinkle plants with calcium orthoarsenate powder, anabadust, nicotine sulphate dust or with tobacco dust mixed with lime, eradicate Brassicáceae weeds and to sow early (Shyogolev, Znamenskij & Bej-Bienko, 1937). There were manual, horse and even aviation sprinklers. Due to these measures, the white mustard yield increased by 40-60%; the brown mustard yield-by 20%; and the spring rapeseed yield-by 70-330%. V.N. Shyogolev (Shyogolev, Znamenskij & Bej-Bienko, 1937) also mentioned the high effectiveness of pyrethrum (a number of its derivatives, synthetic pyrethroids, were later synthesized) and the inexpediency of mechanical measures on large areas and advised to focus attention on agronomic measures (eradication of weeds, optimal timeframe for sowing).
In the 1940s, mandatory weed control was recommended and, upon mass reproduction of flea beetles, sprinkling with a mixture of tobacco dust and lime, pyrethrum and ash, copper acetoarsenite, calcium orthoarsenate, sodium silicofluoride, anabadust or nicotine sulphate dust and ash with kerosene or kreoline was recommended. Sodium orthoarsenate and copper acetoarsenite were used for spraying. A mixture of treacle and starch paste was used as a sticking agent (Moric-Romanova, Berezhkov & Davydov, 1941). N.L. Saharov (Saharov, 1947) demonstrated the necessity for double sprinkling with calcium arsenate: during germination and before anthesis, which completely eliminated the danger of pests. 100% of Phyllotreta spp. flea beetles died within 12-24 hours. For the first time, it was also mentioned about the necessity to get rid of fallen seeds, which are left in great numbers after the harvest of Brassicáceae oilseed crops and a reservoir of flea beetles. V.F. Palij (Palij, 1948) for the first time published data on the effectiveness of Dichlorodiphenyltrichloroethane (DDT) to control Phyllotreta spp. flea beetle numbers: 5% powder at Ramon Research Station in 1946. The population density of flea beetles was 240 insects/m2 before treatment and the sprinkling efficiency was 100% for 12 days. M.G. Alimbekova (Alimbekova, Kukshina & Nikitina, 1949) was first to publish data on the use of hexachlorane against flea beetles in 1947.
In the 1950s, it was recommended to sprinkle crops with hexachlorane or DDT, anabadust or nicotine sulphate dust, calcium orthoarsenate, sodium silicofluoride, pyrethrum and tobacco dust during germination and before anthesis to protect plants against Phyllotreta spp. flea beetles. As to agrotechnical measures, it was recommended to eradicate Brassicáceae weeds, to remove fallen seeds, to sow early (Dobrovolskij, 1950; Velichko, 1951; Kosov, Rameev & Lopaeva, 1952). In addition, for the first time, pre-sowing powdering seeds of Brassicáceae crops with hexachlorane or DDT was recommended to protect seedlings against flea beetles (Zambin, Turaev & Shumilenko, 1953). A.K. Leshenko (Leshenko, 1956) presented data of Uman Agricultural Institute that DDT, 5% powder or with Hexachlorocyclohexane (HCH), 7% powder at a dose of 8-10 kg/ha completely killed flea beetles within 2-3 days.
In the 1960s, it was recommended to powder seeds with hexachlorane; during the growing period, fields were to be sprinkled 2-3 times with DDT, hexachlorane, anabadust, nicotine sulphate dust, metaphos, pyrethrum, sodium silicofluoride or with calcium arsenate (Bardysheva, 1967; Gerasimov & Osnickaya, 1961; Narzikulov, 1968). M.P. Kosmodemyanskij (Kosmodemyanskij & Kulik, 1967) recommended treatment of seeds with hexachlorane, 12% powder within one-two months before sowing and double sprinkling with DDT, 5.5% powder, hexachlorane, 12% powder, chlorophos, 0.2% WP or with metaphos, 2.5% powder during the growing period.
In the 1970s, it was recommended to spray plants with organophosphorus compounds such as chlorophos, metaphos or with malathion and to sprinkle them with calcium arsenate, sodium silicofluoride or with hexachlorane during the growing period (Gorodnij, 1970). W. Teuteberg (Teuteberg, 1973) cites data that in West Germany, in order to control Phyllotreta spp. flea beetle numbers, rapeseeds were first moistened with linseed oil or kerosene and sweat powdered with hexachlorane. In France, Sweden, and Denmark, seeds of Brassicáceae oilseed crops were powdered with hexachlorane (Thompson, 1972; Mattson & Ohlsonn, 1974; Schadlinge des Rapses und ihre Bekampfung, 1974). and, when the pest density was 2-3 beetles/m of the row, the fields were sprayed with mineral-oil emulsion of parathion to protect plants against Phyllotreta spp. flea beetles. In Poland, according to D. Malinowska data (Malinowska, 1974), seeds were powdered with hexachlorane and fields were sprinkled with gamacarbatox to protect plants against Phyllotreta spp. flea beetles. In Canada (Regnault, 1973; Rapeseed Canada’s «Cinderella» Crop, 1974), treatment of seeds with insecticides containing hexachlorane or carbofuran and malathion spraying were used to control Phyllotreta spp. flea beetle numbers. G.I. Konchukovskaya (Konchukovskaya, 1978) recommended applying organophosphorus insecticides Rogor (dimethoate), 40% EC or formothion, 25% EC to the soil concurrently with sowing and using diazinon, 60% EC, Valexon (phoxim), 50% EC, Gardona (tetrachlorvinphos), 50% WP, Elocron (dioxacarb), 50% WP, dilor, 80% WP, phthalophos, 20% EC, phosalone, 35% EC and chlorophos, 80% EC (at a working fluid concentration of 0.1%) during the growing period. These agents were 99-100% effective.
In the 1980s, it was recommended to spray plants with gamma isomer of HCH, 50% WP, polychlorcamphene, chlorophos, 50% EC or with metaphos and to sprinkle with HCH, 12% powder at a flea beetle population density of 2 beetles/m of a row. Concurrently with sowing, it was advisable to apply granulated phosphamide or HCH. Prior to sowing, seeds were treated with HCG, fenthiuram (Gortlevskij & Makeeva, 1983; Ivanov et al., 1985; Shtanko, 1987). Other researchers recommended spraying with phosphamide, chlorophos, dichlorvos, HCH or with metaphos at a population density of 20-30 beetles/m² (Ivanov et al., 1985). V.D. Gajdash recommended the same agents, but at a population density of 5 flea beetles/m² (Gajdash, 1998). A.A. Moskalyova (Moskalyova, 1985) remarked that the efficiency of such organophosphorus insecticides as Actellic (pirimiphos-methyl) and Volaton (phoxim) was high. V.G. Osipov (Osipov, 1986) published data on the high efficiency of seed treatment with fenthiuram or with HCH and application of granular diazinon or phosphamide into the soil, while spraying of seedlings with chlorophos and phosphamide were ineffective. In P.I. Zaycev experiments (Zajcev, 1987), the effectiveness of sumicidin and metaphos was 97% and 85%, respectively. O.N. Serebrennikova (Serebrennikova, 1988) reported that in 1988, as per the list of pesticides permitted for the control of Phyllotreta spp. flea beetles, the application of dimethoate into the soil concurrently with sowing was allowed and seedlings could be sprayed with thiodan and deltamethrin. V.T. Piven (Piven, 1988) recommended spraying of seedlings with a mixture of chlorophos and metaphos, polychlorocamphene or with sumicidin to protect seedlings against Phyllotreta spp. flea beetles. V.G. Osipov (Osipov, 1986) remarked that it was highly effective to apply granular diazinon into the soil concurrently with sowing. N.Z.
Milashenko (Milashenko & Abramov, 1989) was one of the first researchers who recommended protecting seedlings by incrustation of seeds with insecticides.
In the early 1990s, it was recommended to spray plants with sumicidin, thiodan, trichlorometaphos or with malathion and apply ammophos-based dimethoate into the soil concurrently with sowing to protect plants against Phyllotreta spp. flea beetles (Stefanovskij & Majstrenko, 1990). In the mid-1990s, I.M Mazur (Mazur et al., 1997) remarked that spraying seedlings with synthetic pyrethroids such as deltamethrin, cypermethrin, and lambda-cyhalothrin was a reliable way to protect rapeseed and mustard against Phyllotreta spp. flea beetles. These agents are less toxic than organophosphate insecticides and are used at much lower doses.
At the beginning of the 21st century, plant protection is becoming more environmentally friendly. Preference is given to less toxic agents applied at low doses. Pre-sowing protection has been prioritized. Thus, P.D. Sherbak (Sherbak, Sherbak & Majfat, 2001) recommended treatment of seeds with 20% Semafor FC, a triplex insecticide containing biphenthrin, thiamethoxam, the effectiveness of which on seedlings amounted to 85% and the duration of the protective effect was 45 days from the treatment date. V.P. Fedorenko (Fedorenko et al., 2008) recommended protecting seedlings by treating seeds with 20% Chinuk FC, an insecticide containing imidacloprid and beta-cyfluthrin, and 25% Cosmos 250 (fipronil) FC and, if the ETH was exceeded in the germination phase, spraying with synthetic pyrethroids (deltamethrin, 2.5% EC; cypermethrin, 25% EC or with others) was recommended.
Recently, the range of insecticides recommended for protection of Brassicáceae oilseed crops against Phyllotreta spp. flea beetles has become so vast that it is impossible to dwell on each product. Many researchers recommended pre-sowing treatment of seeds or spraying in the phase of 2-4 leaves with one of the permitted insecticides to protect seedlings (Yakovenko, 2005; Lapa et al, 2006; Lazar et al.; 2006; Gordyeyeva, 2007a; Gordyeyeva, 2007b; Zhuravskij & Sekun, 2007; Zhuravskij, Sekun & Skripnik, 2007; Yakovlyev, 2007; Bud’ko, Rovba & Shaganov, 2008; Snizhok, 2008; Sytnyk, 2008; Sekun, 2009; Gordyeyeva, 2010a; Gordyeyeva, 2010b; Abramyk et al., 2010; Ivancova, 2010; Krasilovec, 2010; Yeshenko et al., 2010;. Kasyanov, 2011; Kyforuk et al., 2011; Fedorenko, V.P. & Lugovskij, 2011; Pysarenko, 2011; Lukomec et al., 2012).
The List of Pesticides and Agrochemicals Approved for Use in Ukraine in 2020 includes 17 seed dressers and 79 insecticides for spraying during the growing period to protect oilseed crops against Phyllotreta spp. flea beetles; of them, 32 insecticides (40,5%) are synthetic pyrethroids, 16 insecticides (20,3%) are neonicotinoids and 4 agents (5%) are organophosphorus compounds and 27 insecticides (34,2%) -combined insecticides.
An important role in protecting rapeseed from against Phyllotreta spp. flea beetles is assigned to resistant varieties and hybrids; therefore, in these modern days, breeding for resistance to diseases and pests is one of the main trends in the breeding of oilseeds of the genus Brassica (Gorshkov & Karpachev, 1988; Pilyuk, 2001). A number of pest-resistant varieties have been already created in Europe and the Russian Federation. Of spring rape varieties, according to figures provided by patent holders, Kris (All-Russian Research Institute of Oil Crops, Russia), Lira (All-Russian Research and Design Technological Institute of Rapeseed, Russia), Ribel (Svalof Weibull AB, Sweden), Ural (NPZ-Lembke KG, Germany), and Licolly Germany) are little damaged by Phyllotreta spp. flea beetles (Lychkovskaya, 2009). In Belarus (Pilyuk, 2001), Phyllotreta spp. flea beetles damage the following varieties to a lesser extent: k-330 (Antey), k-4217 (Russia), Liho (Germany), Karat, WW 1490 (Sweden).
Pheromone traps appear to be promising to control flea beetle numbers. Ye. Chonka (Csonka, 2008) suggested that allilisothiocyanate was the best attractant for many species of flea beetles of the genus Phyllotreta (Ph. atra, Ph. nemorum, Ph. undulata, Ph. nigripes, Ph. armoraciae, etc.).
Nowadays, transgenic rapeseed varieties containing the Bacillus thuringiensis (Bt) gene, which makes plants resistant to almost all phytophagous species, are becoming widespread in the world, but in southern China, even this gene does not confer resistance to Ph. striolata.
In Canada, rapeseed is bred to produce varieties that will have pubescence on stems and leaves, which would be similar to that in white mustard (white mustard is less populated with Phyllotreta spp. flea beetles than rapeseed).
A braconid (family Braconidae, subfamily Euphorinae [species is unknown]) is mentioned as a parasitoid of beetles. This parasitoid infests imagoes of all species of the genera Phyllotreta, Chaetocnema and Aphthona. Bright red mite larvae (family Trombidiidae) can infest imagoes (Kostromitin, 1980).
Larvae are infested by two species of parasitoid wasps: Diospilus morosus Reinh (Hymenoptera: Braconidae) and Eulophus sp. (Hymenoptera: Eulophidae) (Tryapicyn, Shapiro & Shepetilnikova, 1982). Both species are ectozoans. In Germany, the parasitoid wasp Tersilochus microgaster and lots of nematodes infest Phyllotreta spp. flea beetles (Hoffman & Schmutterer, 1983).
Two species of nematodes (Howardula phyllotretae and Hexamermis sp.), 1 microsporidium species (Nosema phyllotretae) and 1 gregarine species (Gregarina phyllotretae), which parasitize on Ph. undulate, were detected Turkey (Yaman, Tosun & Aydin, 2009).
Conclusion
The analysis of the literary data indicates that despite the considerable number of the literary sources devoted to the flea beetles, theirs is still a number of its biological and ecological features which are in close connection with the protection measures for controlling it and these measures have not yet been completely clarified. Modern systems of plant protection, consist in developing and implementing the integrated measures that preserve the crops from the harmful organisms while being the safest for the environment, animals and humans. The transition to such integrated systems involves the application of a biological method of pest control, reducing the number of pesticide treatments, the ability to use the preparations of selective action together with the entomophages, etc. An important reserve in this program is the activation and use of natural resources of the beneficial insects (parasitoids and predators) which limit the number of harmful insect-phytophages.
References
Abramyk, M.I. (2010). Zahist ripaka vid hvorob i shkidnikiv. Posibnik hliboroba. Kyiv, Urozhaj, pp:16-25 (in Ukrainian).
Alimbekova, M.G., Kukshina, E.P., Nikitina, T.F. (1949). Glavnejshie vrediteli bolezni i sornyaki selskohozyajstvennyh kultur Gorkovskoj oblasti i mery borby s nimi. Gorkij, OGIZ (in Russian).
Bardysheva, A.M. (1967). Vrediteli i bolezni selskohozyajstvennyh rastenij i mery borby s nimi v usloviyah Magadanskoj oblasti. Magadan, Magadanskoe izd-vo (in Russian).
Bej-Bienko, G.Ya. (1980). Opredelitel nasekomyh Evropejskoj chasti SRSR. Moskva-Leningrad, Nauka (in Russian).
Beleckij, E.N., Stankevich, S.V. (2018). Policiklichnost, sinhronnost i nelinejnost populyacionnoj dinamiki nasekomyh i problemy prognozirovaniya: monografiya. Vena, Premier Publishing s.r.o. Vienna (in Russian).
Bezdelnyj, Yu.M. (1984). Listoedy-vrediteli selsko-hozyajstvennyh kultur. Zashita rastenij: sb. nauch. tr. Novosibirsk, Sibir. otd-nie Vashnii, pp:99-109 (in Russian).
Blomejer, A. (1901). Kultura maslichnyh i voloknistyh rastenij. Besplatnoe pril. k zhurnalu Hozyain. Sankt-Peterburg (in Russian).
Bogdanov-Kat’kov, N.N. (1920). Ogorodnye blohi ili bloshki. Petrograd, Pyataya gosudarstvennaya tipografiya (in Russian).
Bramson, K.P. (1881). Vrednyya nasekomyya i mery dlya borby s nimi. Rukovodstvo dlya hozyaev, narodnih uchitelej i uchitelskih seminarij. Ekaterinoslav, Tipografiya Ya.M. Chausskago (in Russian).
Bud’ko, L.I., Rovba, I.N., Shaganov, I.A. (2008). Raps. Nasha tehnologiya-tradicii kachestva. Minsk, Ravnodenstvie (in Russian).
Chen, C.C., Ko, W.H., Lee, C.L. (1990). Studies on the ecology and control of Phyllotreta striolata (Fab.) (morphology, rearing method, behaviors and host plants). Bulletin of Taichung District Agricultural Improvement Station, 27:37-48.
Csonka, E. (2008). Host plant-related and pheromonal chemical communication of the European Flea Beetle species (Phyllotreta spp., Coleoptera, Chrysomelidae): Thesis of PHd Dissertation. Budapest.
Curikov, M.N. (2009). Zhuki Lipeckoj oblasti. Voronezh, Izdatelsko-poligraficheskij centr Voronezhskogo gosudarstvennogo universiteta (in Russian).
Dedyuhin, S.V., Nikitskij, N.B., Semyonov, V.B. (2005). Sistematicheskij spisok zhestkokrylyh (Insekta, Coleoptera) Udmurtii. Evraziatskij entomologicheskij zhurnal, 4:293-315 (in Russian).
Dobrovolskij, B.V. (1950). Vrediteli polevyh kultur. Rostov nf Donu, Rostizdat (in Russian).
Fasulati, K.K. (1963). Danye o nasekomyh povrezhdayushih ovoshnye kultury Zakarpatya. Nauch. zapiski Uzhgorod. Un-ta, T, 11:118 (in Russian).
Fedorenko, N.V., Stankevich, S.V., Yevtushenko, M.D. (2008). Dinamika chiselnosti osnovnih shkidnikiv ozimogo ripaku zalezhno vid strokiv provedennya zahodiv himichnogo zahistu. Mater. dop. mizhnar. nauk. konf. studentiv, aspirantiv i molodih vchenih. Ekologizaciya stalogo rozvitku agrosferi i noosferna perspektiva informacijnogo suspilstva, p:113 (in Ukrainian).
Fedorenko, V.P., Lugovskij, K.P. (2011). Kontrol hrestocvitih blishok u posivah ozimogo ta yarogo ripaku. Karantin i zahist roslin, 10:7-9 (in Ukrainian).
Fedorenko, V.P. (2008). Zashita rapsa. Zashita i karantin rastenij, 3:69-93 (in Russian).
Filippov, N.A. (1978). Obzor vreditelej ovoshe-bahchevyh kultur i kartofelya v Moldavii. Vrednaya entomofauna ovoshnyh kultur: sb. st. Kishinyov, Shtiica, pp:3-30 (in Russian).
Fokin, A. (2008). Aktualni problemi zahistu ripaku ta sposobi yih podolannya. Propoziciya, 2:68-72 (in Ukrainian).
Gajdash, V.D. (1998). Ripak. Ivano-Frankivsk, Siversiya LTD (in Ukrainian).
Gerasimov, B.A., Osnickaya, E.A. (1961). Vrediteli i bolezni ovoshnyh kultur. 4-e izd. Moskva, Selhozgiz (in Russian).
Gilyarov, M.S. (1964). Opredelitel obitayushih v pochve lichinok nasekomyh. Moskva,Nauka (in Russian).
Gorbatko, K.A. (2010). Zashita rapsa ot vreditelej v zone neustojchivogo uvlazhneniya Centralnogo Predkavkazya: avtoref. dis. kand. s.-h. nauk. Moskva (in Russian).
Gordiyenko, O.V. (2008). Entomokompleks agrobiocenozu grechkovogo polya. O.V. Gordiyenko, Zahist i karantin roslin: mizhvid. Temat Nauk zb Vip, 54:142-145 (in Ukrainian).
Gordyeyeva, O.F. (2007a). Zahist shodiv yarogo ripaku. Agrovisnik. Ukrayina, 1:32 (in Ukrainian).
Gordyeyeva, O.F. (2007b). Efektivnist zastosuvannya insekticidu Decis iz sechovinoyu proti ripakovogo kvitkoyida (Meligethes aeneus F.) na posivah ripaku yarogo v umovah Lisostepu Ukrayini. Mater. vseukr. nauk. konf. molodih uchenih. Ch. 1. Agronomiya. Uman, UDAU, pp:45-46 (in Ukrainian).
Gordyeyeva, O.F. (2010a). Dinamika chiselnosti ripakovogo kvitkoyida (Meligethes aeneus F.) na posivah ripaku ozimogo v umovah Livoberezhnogo Lisostepu Ukrayini. Visn Polt Derzh Agrar Akad, 3:7-9 (in Ukrainian).
Gordyeyeva, O.F. (2010b). Osnovni shkidniki ripaku ta kontrol yih chiselnosti v Livoberezhnomu Lisostepu Ukrayini: avtoref. dis. kand. s.-g. nauk. Harkiv, HNAU (in Ukrainian).
Gorodnij, M.G. (1970). Olijni ta efiroolijni kulturi. Kiyiv, Urozhaj (in Ukrainian).
Gorshkov, V.I., Karpachev, V.V. (1988). Agroekotip yarovogo rapsa dlya uslovij lesostepi CChZ. Novye metody selekcii i sozdanie adaptivnyh sortov s.-h. kultur: rezultaty i perspektivy: tez. dokl. nauch. Sessii, Kirov, pp:113-114 (in Russian).
Gortlevskij, A.A., Makeeva, V.A. (1983). Ozimyj raps. Moskva, Rosselhozizdat (in Russian).
Guseva, O.G., Koval, A.G. (2007). Vidovoj sostav i struktura dominirovaniya zemlyanyh bloshek (Coleoptera: Chrysomelidae, Alticinae) v agrocenozah leningradskoj oblasti. Vestn. zashity rastenij. Sankt-Peterburg-Pushkin, 4:33-40 (in Russian).
Hoffman, G.M., Schmutterer, H. (1983). Parasitare Krankheiten und Schadlinge an landirtschaftlichen Kulturpflancen. Stuttgart, Verlag Eugen Ulmer.
Iskakov, I.S., Krasnikova, V.M. (1991). Vrediteli ogoroda. Alma-Ata, Kajnar (in Russian).
Ivancova, E.A. (2010). Vrediteli gorchicy i rapsa. Pole deyatelnosti, 6:8-11 (in Russian).
Ivanov, O.A. (1985). Vrediteli i bolezni selskohozyajstvennyh kultur v Zapadnoj Sibiri. Novosibirsk, Zapadnosib. kn. izd-vo (in Russian).
Iversen, V.Ye. (1883). Vrednyya polevyya nasekomyya. Opyt prakticheskoj entomologii. Sankt-Peterburg, Izd. F. Pavlenkova (in Russian).
Johnen, A., Klingenhagen, G. (2006). Schedlingskontrolle im Raps. Bekampf-ungsstrategien und Entscheidungshifiten. Raps, 1:18-23.
Johnen, A. (2006). Der Rapserdfloh ist wieder ein Thema! Raps, 1:10-15.
Kalyuga, M.V. (1970) O kormovoj specializacii nekotoryh mnogoyadnyh i ogranichenoyadnyh nasekomyh, povrezhdayushih kormovye krestocvetnye kultury. Zashita rastenij ot vreditelej i boleznej, Tom 127:98-104 (in Russian).
Kanter, L.A., Imyhelova, C.-D.C., Sanzhimitupova, R.D. (1980). Vrediteli kapusty Zapadnogo Zabajkalya. Fauna i ekologiya nasekomyh Zabajkalya: sb.st. Ulan-Ude, BF SO AN SSSR, pp:4-17 (in Russian).
Kasyanov, A.M. (2011). Hrestocviti blishki. Biologichni osoblivosti v umovah centralnogo Lisostepu Ukrayini. Karantin i zahist roslin, 6:11-13 (in Ukrainian).
Kava, L., Stankevych, S. (2013). Shkidniki ripaku gotuyutsya do novogo sezonu. Propoziciya, 3:120-122 (in Ukrainian).
Keppen, F. (1882). Vrednyya nasekomyya. Sochinenie Fyodora Keppena. T.2. Spec. chast. Pryamokrylyya, zhuki i pereponchatokrylyya. Sankt-Peterburg, Tipografiya Imperatorskoj Akad Nauk (in Russian).
Knoll, D. (1997). Pflanzenschutz im Raps Erfahrungen und Empfehlungen aus schleswig-holstcinischer Sicht. Raps, 1:36-38.
Kobahidze, D.N. (1957). Vrednaya entomofauna selskohozyajstvennyh kultur Gruzinskoj SSSR. Tbilisi, Izd-vo AN Gruz. SSR (in Russian).
Kolesnik, L.I. (2007). Osnovni shkidniki kapusti bilogolovoyi u shidnomu lisostepu Ukrayini. Ekologiya i prognoz rozvitku: avtoref dis kand biol nauk. Kharkiv (in Ukrainian).
Konchukovskaya, G.I. (1978). Krestocvetnye bloshki i klubenkovye dolgonosiki v MSSR i mery borby s nimi. Vrednaya entomofauna ovoshnyh kultur: sb.st. Kishinyov, pp:121-125 (in Russian).
Konstantinov, A.S. (1996). Handbook of Palearctic flea beetles (Coleoptera: Chrysomelidae: Alticinae). Contributions on Entomology, International, 1:236-439.
Korolkov, D.M., Durnovo, Z.P. (1926). Vrediteli selskogo hozyajstva i mery borby s nimi v guberniyah srednej polosy RSFSR. Moskva, Izd-vo Mosk Zem Otdela (in Russian).
Kosmodemyanskij, M.P., Kulik, E.N. (1967). Sareptskaya gorchica. Volgograd, Nizhnevolzh. kn. izd-vo (in Russian).
Kosov, N.P., Rameev, H.H., Lopaeva, Z.A. (1952). Maslichnye kultury. Kazan, Tatgosizdat (in Russian).
Kostromitin, V.B. (1980). Krestocvetnye bloshki. Moskva, Kolos (in Russian).
Kovalchuk, G.M. (1987). Ripak ozimij-cinna olijna i kormova kultura. Kiyiv, Urozhaj (in Ukrainian).
Krasilovec, Yu. (2011). Dva aspekti zahistu ripaku. Agrobiznes sogodni, 10:24-28 (in Ukrainian).
Krasilovec, Yu.G. (2010). Naukovi osnovi fitosanitarnoyi bezpeki polovih kultur. Harkiv, Magda LTD, pp:416 (in Ukrainian).
Krasilovec, Yu.G., Kuzmenko, N.V., Litvinov, A.Ye., Stankevich, S.V. (2011). Efektivnist protrujnikiv pri zahisti yarogo ripaku vid hrestocvitih blishok (Phyllotreta spp.) na doslidnih polyah institutu roslinnictva im. V.Ya. Yur’yeva NAANU. Biologichne riznomanittya ekosistem i suchasna strategiya zahistu roslin: Mater. Mizhnar. nauk.-prakt. konf. do 90-richchya z dnya narodzhennya d. b. n., prof. Litvinova B. M. 29-30 veresnya 2011 r. Harkiv, HNAU, pp:50-52 (in Ukrainian).
Kyforuk, I.M. (2011). Rekomendaciyi z viroshuvannya girchici v umovah Prikarpattya. Posibnik hliboroba. Kyiv, Urozhaj, pp:216-222 (in Ukrainian).
Laba, Yu.R., Sytnyk, I.D. (2006). Zmina chiselnosti shkidlivih komah pid diyeyu himichnih preparativ na riznih sorah yarogo ripaku. Agrarna nauka i osvita, T, 7:70-73 (in Ukrainian).
Lapa, O.M. (2006). Tehnologiya viroshuvannya ta zahistu ozimogo ripaku. Kyiv, Kolobig (in Ukrainian).
Lazar, T.I. (2006). Intensivna tehnologiya viroshuvannya ozimogo ripaku v Ukrayini. Kyiv: Universal-Druk (in Russian).
Lee, C.F. (2011). A Review of Phyllotreta Chevrolat in Taiwan (Coleoptera: Chrysomelidae: Galerucinae: Alticini). Zoological Studies, 50:525-533.
Legatov, V. (1929). Opredelitel povrezhdenij rastenij. Tambov, Tambov. Stanciya Zashity Rastenij, (in Russian).
Leshenko, A.K. (1956). Olijni ta efiroolijni kultury. Kyiv, Derzhsilgospvidav URSR (in Ukrainian).
Levkovich, E.V., Levkovich, V.G. (2006). Zhuki Penzenskoj oblasti. Izvestiya PGPU im. V.G. Belinskogo. Estestvennye nauki. Vypusk posvyashen 60-letiyu Estestvenno-Geograficheskogo Fakulteta, 1:100-104 (in Russian).
Lhagva, Zh. (1971). Osnovnye vrediteli kapusty v usloviyah Lesostepnoj zony MNR i sistema meropriyatij po borbe s nimi: avtoref. dis. kand. s.-h. nauk. Leningrad-Pushkin, (in Russian).
Lindeman, K. (1866). Ocherki iz zhizni zhukov. Moskva, Tip. Grachyova, (in Russian).
Lopatin, I.K. (1986). Zhuki-listoedy fauny belorussii i pribaltiki: opredelitel. Minsk, Vysh shk (in Russian).
Lukomec, V.M. (2012). Zashita rapsa. Zashita i Karantin Rastenij, 1:53-85 (in Russian).
Lychkovskaya, I.Yu. (2009). K ocenke povrezhdaemosti yarovogo rapsa krestocvetnymi bloshkami. V mezhdunarodnaya konferenciya molodyh uchenyh i specialistov: tezisy dokladov konf, pp:128-130 (in Russian).
Malinowska, D. (1974). Skutekznosc pestycydow zalekanych do ochrony rzepacu ozimego w swietle doswiadczen WSKIOR w Lublinie. Ochrona Rosl, 18:11-12.
Manaenkova, T.I. (1991). Ustojchivost yarovogo rapsa k krestocvetnym bloshkam (Phyllotreta spp.) i rapsovomu cvetoedu (Meligethes aeneus F.) avtoref. dis. kand. s.-h. nauk. Leningrad, (in Russian).
Marchenko, A.B. (2011). Shkidliva entomofauna kapustyanih agrocenoziv ta yiyi ekologichni vzayemovidnosini z fitopatogennimi bakteriyami. Karantin i Zahist Roslin, 1:13-15 (in Ukrainian).
Mattson, E., Ohlsonn, J. (1974). Varraps och Varrybs. Akktuellt fran Lantbrukshogskolan.
Mazur, I.A. (1997). Nadijnij zahist ripaka ta girchici vid hvorob ta shkidnikiv. Nauk.-tehn. byul. In-tu olijnih kultur, Vip. 2, Zaporizhzhya, pp:194-196 (in Ukrainian).
Melnichuk, V.T. (1996). Tehnologiya viroshuvannya i vikoristannya ripaku. Ivano-Frankivsk (in Ukrainian).
Melnik, A.V. (2007). Agrobiologichni osoblivosti viroshuvannya sonyashniku ta ripaku yarovogo v umovah Pivnichno-Shidnogo LisostepuUkrayini. Sumi, Universitetska Kniga (in Ukrainian).
Milashenko, N.Z., Abramov, V.F. (1989). Tehnologiya vyrashivaniya i ispolzovaniya rapsa i surepicy. Moskva, VO Agropromizdat (in Russian).
Minkevich, I. A., Borisovskij, V.E. (1949). Maslichnye kultury. Moskva, Selhozgiz (in Russian).
Moric-Romanova, Z.E., Berezhkov, R.P., Davydov, P.N. (1941). Vrediteli i bolezni selskohozyajstvennyh rastenij Zapadnoj Sibiri i borba s nimi. Novosibirsk, OGIZ (in Russian).
Moskalyova, A.A. (1985). Vidovoj sostav vreditelej rapsa, mery borby s nimi (Leningradskaya oblast). Integrirovannaya zashita rastenij ot vreditelej i boleznej: sb. nauch. tr. Leningrad, pp;24-26 (in Russian).
Narzikulov, M. (1968). Vrediteli i bolezni selskohozyajstvennyh kultur Tadzhikistana. Izd. 2-e, per. i dop. Dushanbe, Irfon (in Russian).
Obzor vrednyh nasekomyh Kupyanskago uezda po nablyudeniyam 1905 goda (1906). Harkov, Tipografiya Pechatnoe Delo (in Russian).
Osipov, V.G. (1986). Mery borby s krestocvetnymi bloshkami na kormovih krestocvetnyh kulturah. Zashita rastenij: sb. nauch. tr. Vyp. XI, pp:17-22 (in Russian).
Osmolovskij, G.E. (1972). Vrediteli kapusty. Leningrad, Kolos, (in Russian).
Otchyot Entomologicheskago Byuro Harkovskogo gubernskogo zemstva za 1913 god. (1913). S prilozheniem obzora vreditelej, deyatelnosti uezdnyh zemstv po borbe s vreditelyami i zhurnala soveshaniya o lugovom motylke. Harkov, Tovarishestvo Pechatnya S.P. Yakovleva (in Russian).
Palij, V.F., Avanesova, G.A. (1975). Zemlyanye bloshki Coleoptera, Chrysomelidae, Halticinae: opredelitel rodov i vrednyh vidov. AN UzSSR, In-t zoologii i parazitologi. Tashkent, Fan (in Russian).
Palij, V.F. (1948). Kak vesti borbu s vreditelyami selskohozyajstvennyh rastenij. Voronezh, Voronezhskoe obl.-e izd-vo (in Russian).
Palij, V.F. (1954). Smena pitayushih rastenij u zemlyanyh bloshek (Halticinae, Chrysomelidae, Coleoptera) v raznyh geograficheskih usloviyah Palearktiki. Tez. dok. III ekologich. konf. Kyiv, pp:196-199 (in Russian).
Palij, V.F. (1962). Rasprostranenie, ekologiya i biologiya zemlyanyh bloshek fauny SSSR/V.F. Palij-Frunze, Izd-vo AN Kirgiz. SSR (in Russian).
Perelik pesticidiv ta agrohimikativ dozvolenih do vikoristannya v Ukrayini na 2020 rik (2020). Kyiv, Yunivest marketing (in Ukrainian).
Pilyuk, Ya.E. (2001). Rezultaty izucheniya genofonda yarovogo rapsa v Belarusi. Nauka-proizvodstvu: mat. 4-j mezhdunar. Nauchno-proekt Konf Grodno, pp:35-38 (in Russian).
Piven, V.T. (1988). Zashita rapsa i surepicy ot vreditelej i boleznej. Tehnicheskie Kultury, 3:23-24 (in Russian).
Plavilshikov, N.N. (1994). Opredelitel nasekomyh. Kratkij opredelitel naibolee rasprostranyonnyh nasekomyh Evropejskoj chasti Rossii. Moskva, Topikal (in Russian).
Podkopayev, A. (1933). Shkidniky gorodu i zahody borotby z nymy. Kharkiv, Derzhsilgospvidav (in Ukrainian).
Purievich, K.A. (1893). Vazhnejshie vragi zemledeliya. Kyiv, Tipografiya Petra Barskago (in Russian).
Putele, V. (1970). Vidovoj sostav zemlyanyh bloshek v Latvijskoj SSR. Materialy 7-go Pribalt. soveshaniya po zashite rastenij Vrediteli selskohoyajstvennyh i lesnyh rastenij i mery borby s nimi. Ch.I. Elgava, pp:17-20 (in Russian).
Pyatakova, V.D. (1928). Ogorodnye bloshaki. Mleev (in Russian).
Pysarenko, V.M., Gordyeyeva, O.F. (2009). Shkidlivist osnovnih vidiv fitofagiv ripaku yarogo ta ozimogo v Lisostepu Ukrayini. Visnik Polt Derzh Agrar Akad, 2:5-9 (in Ukrainian).
Pysarenko, V.M. (2011) Sideralni kultury Poltava: Simon (in Ukrainian).
Rapeseed Canada’s Cinderella Crop. (1974). Published by rapeseed association of Canada.
Razumov, V.P. (1971) Entomofagi vreditelej kapusty v usloviyah Gorkovskoj oblasti: avtoref dis kand biol nauk Leningrad-Pushkin (in Russian).
Regnault, Y. (1973). La grosse altise du colsa Producteur agr franc, 49:135.
Saharov, N.L. (1934). Vrediteli gorchicy i borba s nimi Saratov, Saratovskoe kraevoe gos izd-vo, p:120.
Saharov, N.L. (1947). Vrednye nasekomye Nizhnego Povolzhya Saratov, OGIZ; Saratovskoe oblastnoe izd-vo (in Russian).
Samedov, N.G. (1963). Fauna i biologiya zhukov vredyashih selskohozyajstvennym kulturam v Azerbajdzhane Baku, Izd-vo AN Azerb SSSR (in Russian).
Satalkina, G.I., Ancupova, T.E. (1993). Vliyaie povrezhdenij krestocvetnyh bloshek i klopov na fiziologobiohimicheskie processy v listyah rapsa Tr Kuban gos agrar un-ta, 332:165-168 (in Russian).
Schadlinge des Rapses und ihre Bekampfung. (1974). Merkblatt des Pflanzenschutzes, 6:1-8.
Sekun, M.P. (2009). Zahist posiviv yarogo ripaku vid shkidnikiv Agronom, 2:80-84 (in Ukrainian).
Sekun, M.P. (2008) Tehnologiya viroshuvannya i zahistu ripaku Kyiv, Globus-Print (in Ukrainian).
Semakov, V.V. (1966). Vrediteli krestocvetnyh kultur Kamchatki i borba s nimi Petropavlovsk-Kamchatskij, Dalnevost-e kn-e izd-vo (in Russian).
Serebrennikova, O.N. (1988) Problemy zashity rapsa i surepicy ot krestocvetnyh bloshek Tehnicheskie Kultury, 3:20-22 (in Russian).
Sergeev, M.E. (2007). Zemlyanye bloshki (Coleoptera, Chrysomelidae, Alticinae) lesostepnoj zony Ukrainy Tezi dop VII z’yizdu Ukr entomol t-va Nizhin, p:118 (in Russian).
Shapiro, D.S. (1964). Fauna zemlyanyh bloshek roda Phyllotreta Stephens Evropejskoj chasti SSSR (Coleoptera, Chrysomelidae, Subfarm Halticinae) Voprosy genetiki i zoologi Harkov, pp:82-107 (in Russian).
Shejgerevich, G.I. (1988). Raps na korm i semena: sbornik Minsk, Uradzhaj (in Russian).
Sherbak, P.D., Sherbak, O.P., Majfat, O.V., (2001). Efektivnij zahist ripaka vid shkidnikiv Nauk-tehn byul In-tu olijnih kultur UAAN, Vip, 6:185-187 (in Ukrainian).
Shtanko, A.V. (1987). Raps-vysokourozhajnaya belkovaya kultura Petrozavodsk, Kareliya (in Russian).
Shtejnberg, P.N. (1907). Vrednyya nasekomyya i ispytannye sposoby borby s nimi. PN Shtejnberg-Sakt-Peterburg, Knigoizd-vo P P Sojkina (in Russian).
Shutak, V.I. (1973). Massovye vrednye vidy zemlyanyh bloshek Bashkirii i Yuzhnogo Preduralya Nauka-proizvodstvu: sb Tr, pp:85-88 (in Russian).
Shyogolev, V.N. (1960). Opredelitel nasekomyh po povrezhdeniyam kulturnyh rasteni Moskva, Selhozgiz (in Russian).
Shyogolev, V.N., Znamenskij, A.V., Bej-Bienko, G.Ya. (1937). Nasekomye vredyashie polevym kulturam 2-e izd, per i dop Moskva-Leningrad, Selhozgiz (in Russian).
Skripnik, O.V., Zhuravskij, V.S. (2004). Sistema himichnogo zahistu yarogo ripaku vid shkidnikiv Integrovanij zahist roslin na pochatku HHI stolittya Kyiv, pp:299-303 (in Ukrainian).
Smirnov, A.P. (2009). Vidovoj sostav i dinamika chislennosti krestocvetnyh bloshek na stolovih koreneplodah v Leningradskoj oblasti Vestnik Zashity Rastenij, Sankt-Peterburg-Pushkin, 2:38-43 (in Russian).
Snizhok, O.V. (2008). Efektivnist himichnogo zahistu shodiv ozimogo ripaku vid shkidnikiv v zahidnomu Lisostepu Ukrayini Zahist i karantin roslin: mizhvid temat zb, Vip, 54:365-370 (in Ukrainian).
Solovyova, A.A. (1970) Vrediteli selskohozyajstvennyh kultur Kirgizii Frunze, Kyrgyzstan (in Russian).
Soroka, J, Elliot, B. (2011). Managing flea beetles in canola prairie soils & crops. Journal Insects and Diseases, 4:1-7.
Spisok nasekomyh (i nekotorh drugih nizshih zhivotnyh) naibolee vrednyh v hozyajstvennom otnoshenii. (1908). Izd 3-e, dop Sankt-Peterburg, Tipografiya M Merkusheva (in Russian).
Stankevich, S.V., Fedorenko, N.V. (2009). Dominiruyushie vidy vreditelej yarovogo rapsa i gorchicy i ih hozyajstvennoe znachenie Mater HI mezhdunar nauch-prakt ekol konf. Vidovye populyacii i soobshestva v estestvennyh i antropogenno transformirovannyh landshaftah: sostoyanie i metody ego diagnostiki, 20-25 Sentyabrya 2010 g, p:189 (in Russian).
Stankevich, S.V., Fedorenko, N.V. (2011) Effektivnost insekticidov pri zashite yarovogo rapsa ot glavnejshih vreditelej do cveteniya Nauchnye vedomosti. Belgorodskogo Gosudarstvennogo Universiteta Seriya Estestvennye Nauki, 3:91-94 (in Russian).
Stankevich, S.V., Vilna, V.V. (2012) Vrediteli generativnyh organov yarovogo rapsa i gorchicy v vostochnoj Lesostepi Ukrainy Mater HII mezhdunar nauch-prakt ekol konf. Strukturno-funkcionalnye izmeneniya v populyaciyah i soobshestvah na territoriyah s raznym urovnem antropogennoj nagruzki. Oktyabrya 2012 g, pp:207-208 (in Russian).
Stankevich, S.V. (2011d). Vrednaya entomofauna yarovogo rapsa i gorchicy iz otryada zhestkokrylyh (Coleoptera) Mater VIII Vserossijskoj nauch-prakt konf (s mezhdunar uchastiem). Tobolsk nauchnyj-2011. 11-12 Noyabrya 2011 g, pp:69-70 (in Russian).
Stankevich, S.V. (2012). Rasteniya-rezervatory vreditelej maslichnyh krestocvetnyh kultur Byuleten nauchnyh rabot BelSHA, 32:22-32 (in Russian).
Stankevich, S.V. (2015). Sezonnaya dinamika chislennosti rapsovogo cvetoeda na yarovom rapse i gorchice v vostochnoj lesostepi Ukrainy Zashita rastenij Sbornik nauchnyh trudov, 39:197-203 (in Russian).
Stankevich, S.V., Beleckij, E.N., Zabrodina, I.V. (2019). Ciklicheski-nelinejnaya dinamika prirodnyh sistem i problemy prognozirovaniya. Vankuver, Accent Graphics Communications & Publishing Vankuver, p:232 (in Russian).
Stankevych, S.V., Yevtushenko, M.D., Vilna, V.V. (2020). Dominant pests of spring rape and mustard in the eastern Forest-Steppe of Ukraine and ecologic protection from them: monograph Kharkiv, Publishing House IIvanchenko.
Stankevych, S.V., Fedorenko, N.V. (2009). Protruyuvannya nasinnya yak pershij zahid zahistu yarogo ripaku vid shkidnikiv Tezi dop Mizhnar nauk konf studentiv, aspirantiv i molodih vchenih. Ekologizaciya stalogo rozvitku agrosferi i noosferna perspektiva informacijnogo suspilstva. 1-2 Zhovtnya 2009, p:117 (in Ukrainian).
Stankevych, S.V., Fedorenko, N.V. (2010). Shkidliva entomofauna ripaku j girchici na doslidnomu poli HNAU im V V Dokuchayeva Tezi dop entomol nauk konf, prisvyachenoyi 60-j richnici stvorennya Ukrayinskogo entomologichnogo tovaristva. Suchasni problemi entomologiyi. 12-15 Zhovtnya 2010 r, pp:169 (in Ukrainian).
Stankevych, S.V., Vilna, V.V. (2012). Zalezhnist laboratornoyi shozhosti nasinnya yarogo ripaku vid peredposivnogo obrobitku insektofungicidnimi sumishami Introdukciya, selekciya ta zahist roslin Mater III mizhnar nauk konf 25-28 veresnya 2012 r, pp:169 (in Ukrainian).
Stankevych, S.V. (2010). Zahist girchici biloyi vid ripakovogo kvitkoyida na doslidnomu poli HNAU im V V Dokuchayeva Tezi dop Mizhnar nauk konf studentiv, aspirantiv i molodih vchenih. Ekologizaciya stalogo rozvitku agrosferi i noosferna perspektiva informacijnogo suspilstva. 4-5 zhovtnya 2010 r, pp:104-105 (in Ukrainian).
Stankevych, S.V. (2011a). Biologichni osoblivosti ripakovogo kvitkoyida v umovah Harkivskoyi oblast. Aktualni problemi prirodnichih ta gumanitarnih nauk u doslidzhennyah molodih vchenih. Rodzinka-2011 Zb mater HIII Vseukr nauk konf molodih vchenih Seriya prirodnichi ta komp’yuterni nauki 14-15 kvitnya 2011 r, pp:91-93 (in Ukrainian).
Stankevych, S.V. (2011b). Fitofagi olijnih kapustyanih kultur v umovah Harkivskogo rajonu Mater V Vseukr nauk-prakt konf molodih uchenih. Ekologichni problemi silskogospodarskogo virobnictva. 21-24 Chervnya 2011 r, pp:178-179 (in Ukrainian).
Stankevych, S.V. (2011c). Biologichni osoblivosti hrestocvitih blishok ta ripakovogo kvitkoyida v umovah Harkivskoyi oblasti Fundamentalni ta prikladni doslidzhennya v biologiyi: Mater II Mizhnar nauk konf studentiv, aspirantiv ta molodih uchenih 19-22 veresnya 2011 r, pp:62-63 (in Ukrainian).
Stankevych, S.V. (2012a). Roslini-rezervatori ripakovogo kvitkoyida Mater X mizhnar nauk konf studentiv ta molodih naukovciv. Shevchenkivska vesna 2012. 19-23 Bereznya 2012 r, pp:289-290 (in Ukrainian).
Stankevych, S.V. (2012b). Roslini-rezervatori kapustyanih blishok Suchasni problemi biologiyi, ekologiyi ta himiyi Zb mater III Mizhnar nauk-prakt konf, prisvyachenoyi 25-richchyu biol fak-tu 11-13 travnya 2012 r, pp:167-168 (in Ukrainian).
Stankevych, S.V. (2012c). Vrediteli vshodov maslichnyh krestocvetnyh kultur v usloviyah vostochnoj Lesostepi Ukrainy Mater XIV sezda Russkogo entomologicheskogo obshestva 27 avgusta-1 sentyabrya 2012 g, p:408 (in Russian).
Stankevych, S.V. (2012d). Vidovoj sostav kompleksa krestocvetnyh bloshek v vostochnoj Lesostepi Ukrainy Mater XV Mezhdunar nauch-prakt konf. Sovremennye tehnologii s-h proizvodstva. 18 maya 2012 g Ch 1, pp:173-175 (in Russian).
Stankevych, S.V. (2012e). Bagatorichna sezonna dinamika chiselnosti kapustyanih blishok v umovah Harkivskogo rajonu Dinamika bioriznomanittya 2012: zb Nauk Prac, pp:108-109 (in Ukrainian).
Stankevych, S.V. (2012f). Efektivnist zahistu ripaku j girchici vid ripakovogo kvitkoyidu na doslidnomu poli HNAU im V V Dokuchayeva Tezi dop Mizhnar nauk konf studentiv, aspirantiv i molodih vchenih. Ekologizaciya stalogo rozvitku agrosferi i noosferna perspektiva informacijnogo suspilstva. 3-5 Zhovtnya 2012 r, pp:170-171 (in Ukrainian).
Stankevych, S.V. (2012g). Specializovani shkidniki ripaku j girchici u Harkivskomu rajoni Materiali mizhnarodnoyi naukovo-praktichnoyi Internet-konferenciyi. Prikladna nauka ta innovacijnij shlyah rozvitku nacionalnogo virobnictva. 4-5 Zhovtnya 2012 r, Ternopil, pp:47-48 (in Ukrainian).
Stankevych, S.V. (2012h). Zastosuvannya mikrobiopreparatu aktofit v poyednanni z insekticidom biskaya proti ripakovogo kvitkoyidu u fenofazu zhovtogo butonu Visnik Harkivskogo nacionalnogo agrarnogo universitetu im V.V Dokuchayeva Seriya "Fitopatologiya ta entomologiya", 12:115-122 (in Ukrainian).
Stankevych, S.V. (2014) Yakisni pokazniki nasinnya ripaku yarogo zalezhno vid protruyuvannya ta poshkodzhennya lichinkami ripakovogo kvitkoyida Visnik HNAU im V V Dokuchayeva Ser "Fitopatologiya ta entomologiya", 8:114-120 (in Ukrainian).
Stankevych, S.V. (2015). Zmina paradigmi u zahisti olijnih kapustyanih kultur vid hrestocvitih blishok za 130 rokiv Visn Hark nac agrar un-tu im V V Dokuchayeva Seriya "Fitopatologiya ta entomologiya", 1:151-175 (in Ukrainian).
Stankevych, S.V., Baidyk, H.V., Lezhenina, I.P. (2019) Wandering of mass reproduction of harmful insects within the natural habitat. Ukrainian Journal of Ecology, 9:578-583.
Stankevych, S.V., Biletskyj, Ye.M., Golovan, L.V. (2020). Polycyclic character, synchronism and nonlinearity of insect population dynamics and prognostication problem: monograph Kharkiv: Publishing House I Ivanchenko.
Stankevych, S.V., Biletskyj, Ye.M., Zabrodina, I.V. (2020). Prognostication algorithms and predictability ranges of mass reproduction of harmful insects according to the method of nonliner dynamics. Ukrainian Journal of Ecology, 10:37-42.
Stankevych, S.V., Biletskyj, Ye.M., Zabrodina, I.V. (2020). Cycle populations dynamics of harmful insects. Ukrainian Journal of Ecology, 10:147-161.
Stankevych, S.V., Biletskyj, Ye.M., Zabrodina, I.V. (2020). Prognostication in plant protection Review of the past, present and future of nonliner dynamics method. Ukrainian Journal of Ecology, 10:225-234.
Stankevych, S.V., Teslina, V.V., Ozhga, I.I. (2010). Vnesennya dobriv yak neobhidnij element integrovanogo zahistu olijnih kapustyanih kultur Tezi dop Mizhnar nauk konf studentiv, aspirantiv i molodih vchenih. Ekologizaciya stalogo rozvitku agrosferi i noosferna perspektiva informacijnogo suspilstva. 4-5 Zhovtnya 2010 r, pp:102-103 (in Ukrainian).
Stankevych, S.V., Vasylieva, Yu.V., Golovan, L.V. (2019). Chronicle of insect pests massive reproduction. Ukrainian Journal of Ecology, 9:262-274.
Stankevych, S.V., Vilna, V.V., Zabrodina, I.V. (2021). Efficiency of chemical protection of spring rape and mustard from cruciferous bugs. Ukrainian Journal of Ecology, 11:52-59.
Stankevych, S.V., Vilna, V.V., Zabrodina, I.V. (2021). Harmfulness of cruciferous bugs. Ukrainian Journal of Ecology, 11:417-428.
Stankevych, S.V., Yevtushenko, M.D., Vilna, V.V. (2019). Integrated pest management of flea beetles (Phyllotreta spp) in spring oilseed rape (Brassica napus L). Ukrainian Journal of Ecology, 9:198-207.
Stankevych, S.V., Yevtushenko, M.D., Vilna, V.V. (2019). Efficiency of chemical protection of spring rape and mustard from rape blossom beetle. Ukrainian Journal of Ecology, 9:584-598.
Stankevych, S.V., Yevtushenko, M.D., Zabrodina, I.V. (2020). Pests of oil producing cabbage crops in the eastern forest-steppe of Ukraine. Ukrainian Journal of Ecology, 10:223-232.
Stankevych, S.V., Yevtushenko, M.D., Zabrodina, І.V. (2019). V.V Dokuchaiev Scientific school of Kharkiv National Agrarian University and development agricultural entomology in XIX-XXI centuries. Ukrainian Journal of Ecology, 9:170-178.
Stefanovskij, V.V., Majstrenko, G.S. (1990). Intensivnaya tehnologiya proizvodstva rapsa Moskva, Rosagropromizdat (in Russian).
Susidko, V.I., Pisarenko, V.N. (1991). Zashita sadovyh i ovoshnyh kultur bez primeneniya pesticidov Moskva, Gosagropromizdat (in Russian).
Sytnyk, I.D. (2008). Tehnologiya viroshuvannya ozimogo i yarogo ripaka Posibnik hliboroba, pp:77-90 (in Ukrainian).
Teuteberg, W. (1973). Umstellung Im Rapsanbau Aktuelles Acker-Pflanzenbau Oldenburg, 1:57-67.
Thompson, K.F. (1972). Cytoplasmik malesterility in Oilseed rape Heredity, 29:253-257.
Troickij, N.I., Shegolev, V.N. (1934). Opredelitel povrezhdenij kulturnyh rastenij Moskva-Leningrad, Selhozgiz (in Russian).
Tryapicyn, V.A., Shapiro, V.A., Shepetilnikova, V.A. (1982). Parazity i hishniki vreditelej selskohozyajstvennyh kultur Izd 2-e, per i dop Leningrad, Kolos (in Russian).
Vasilev, V.P. (1988). Vrediteli selskohozyajstvennyh kultur i lesnyh nasazhdenij T 2 Vrednye chlenistonogie, pozvonochnye Izd 2-e ispr i dop Kyiv, Urozhaj (in Russian).
Velichko, V.V. (1951). Belaya gorchica v nechernozyomnoj polose Moskva, Selhozgiz (in Russian).
Volker, H.P. (2003). Raps krankheiten, schadlinge, schadpflanyen gelsenkirchen-buer, Verlag Th Mann.
Yakovenko, T.M. (2005). Olijni kulturi Ukrayini Kyiv, Urozhaj (in Ukrainian).
Yakovlyev, R.V. (2007). Efektivnist insekticidiv pri riznih metodah yih zastosuvannya proti shkidnikiv shodiv girchici Nauk-tehn byul IOK UAAN, Vip, 12:263-266 (in Ukrainian).
Yaman, M., Tosun, O., Aydin, C. (2009). Occurrence of the Pathogens and Parasites of Phyllotreta undulata (Coleoptera: Chrysomelidae) in Turkey Turk J Zool, 33:139-146.
Yeshenko, V.O. (2010). Tehnologiya viroshuvannya ripaka yarogo v Lisostepu Ukrayini Uman, Vidavec. Sochinskij (in Ukrainian).
Yevtushenko, M.D., Stankevych, S.V., Vilna, V.V. (2014). Hrestocviti blishki, ripakovij kvitkoyid na ripaku yaromu j girchici u Shidnomu Lisostepu Ukrayini, Harkiv, Majdan (in Ukrainian).
Yevtushenko, M.D., Vilna, V.V., Stankevich, S.V. (2016). Hrestocviti klopi na ripaku yaromu j girchici u Shidnomu Lisostepu Ukrayini, Harkiv, FOP Brovin OV (in Ukrainian).
Yevtushenko, M.D., Stankevych, S.V. (2009). Fitofagi ozimogo ta yarogo ripaku j girchici na doslidnomu poli HNAU im V V Dokuchayeva Zb dop VIII mizhnar nauk konf aspirantiv i studentiv. Ohorona navkolishnogo seredovisha ta racionalne vikoristannya prirodnih resursiv. 14-16 Travnya 2009 r, 2:14-15 (in Ukrainian).
Yevtushenko, M.D., Stankevych, S.V. (2010). Deyaki biologichni osoblivosti ripakovogo kvitkoyida ta efektivnist insekticidiv u fenofazu zhovtogo butona Visnik Harkivskogo nacionalnogo agrarnogo universitetu im V V Dokuchayeva Seriya "Fitopatologiya ta entomologiya", 1:40-47 (in Ukrainian).
Yevtushenko, M.D., Stankevych, S.V. (2011). Efektivnist protrujnikiv pri zahisti shodiv yarogo ripaku vid kompleksu hrestocvitih blishok Visnik Harkivskogo nacionalnogo agrarnogo universitetu im V V Dokuchayeva Seriya "Fitopatologiya ta entomologiya", 9:63-68 (in Ukrainian).
Yevtushenko, M.D., Fedorenko, N.V., Stankevych, S.V. (2007). Osnovni shkidniki olijnih kapustyanih kultur na doslidnomu poli HNAU im V.V Dokuchayeva Mater dop Mizhnar nauk konf studentiv, aspirantiv i molodih vchenih. Ekologizaciya stalogo rozvitku agrosferi i noosferna perspektiva informacijnogo suspilstva. 3-5 Zhovtnya 2007 r Harkiv, HNAU, pp:239-240 (in Ukrainian).
Yevtushenko, M.D., Fedorenko, N.V., Stankevych, S.V. (2008). Vidovij sklad ta dinamika chiselnosti osnovnih shkidnikiv olijno-kapustyanih kultur u Harkivskij oblasti Visnik Harkivskogo nacionalnogo agrarnogo universitetu im V.V Dokuchayeva Seriya "Entomologiya ta fitopatologiya", 8:47-54 (in Ukrainian).
Yevtushenko, M.D., Stankevych, S.V., Fedorenko, N.V. (2009). Efektivnist insekticidiv pri zahisti yarogo ripaku vid blishok (Phylotretta spp) ta klopiv (Eurydema spp) do cvitinnya Visnik Harkivskogo nacionalnogo agrarnogo universitetu im V.V Dokuchayeva Seriya "Entomologiya ta fitopatologiya", 8:39-43 (in Ukrainian).
Yevtushenko, N.D., Stankevych, S.V. (2011). Roslini-rezervatori osnovnih shkidnikiv olijnih kapustyanih kultur Visti Harkivskogo entomologichnogo tovaristva T XIX Vip 2, pp:71-76 (in Ukrainian).
Yevtushenko, N.D., Stankevich, S.V. (2012). Sezonnaya dinamika chislennosti rapsovogo cvetoeda, Meligethes aeneus (F, 1775) (Coleoptera: Nitidulidae) na yarovom rapse i gorchice v Harkovskom rajone Izvestiya Harkovskogo entomologicheskogo obshestva, T XX Vyp, 2:65-68 (in Russian).
Zajcev, P.I. (1987). Sistema zashity yarovogo rapsa Zashita rastenij, 8:30 (in Russian).
Zambin, I.M., Turaev, N.S., Shumilenko, E.P. (1953). Vrediteli i bolezni selskohozyajstvennyh kultur Sverdlovsk, Kn izd-vo (in Russian).
Zhao, Y. (2008). Phyllotreta striolata (Coleoptera: Chrysomelidae): Arginine kinase cloning and RNAi-based pest control. European Journal of Entomology, 105:815-822.
Zhuravskij, V.S., Sekun, M.P. (2007). Himichnij metod obmezhennya chiselnosti osnovnih shkidnikiv yarogo ripaku Nauk-tehn byul In-tu olijnih kultur UAAN, Vip 12, Zaporizhzhya, pp:188-192 (in Ukrainian).
Zhuravskij, V.S., Sekun, M.P., Skripnik, O.V. (2007). Insekticidi proti hrestocvitih blishok na yaromu ripaku Zahist i karantin roslin: mizhvid temat nauk zb Vip, 53:59-63 (in Ukrainian).
Author Info
S. Stankevych1*, I.V. Zabrodina1, M. Filatov1, L. Sirous1, D. Yushchuk1, V. Melenti1, K. Novosad1, L. Kava2, H. Kosylovych3, Yu. Holiachuk3, I. Derevyanko1, K. Katerynchuk4, I. Kovalenko4, O. Koval4 and S. Kyrenko52National University of Life and Environmental Sciences of Ukraine, Kyiv, 03041, Ukraine
3Lviv national agricultural university, 1 Volodymyra Velykogo Str., Dublyany, Zhovkva district, Lviv region, 80381, Ukraine
4Kyiv National University of Technologies and Design, 2 Nemirovich-Danchenko Str., Kyiv, 01011, Ukraine
5State University of Infrastructure and Technology, 9 Kyrylivska Str., Kyiv, 04071, Ukraine
Citation: Stankevych, S., Zabrodina, I.V., Filatov, ?., Sirous, L., Yushchuk, D., Melenti, V., Novosad, K., Kava, L., Kosylovych, H., Holiachuk, Yu., Derevyanko, I., Katerynchuk, K., Kovalenko, I., Koval, O., Kyrenko, S. (2021). Flea beetles (Phyllotreta spp.): Species composition, range, bioecological features, harmfulness and protection measures: Review. Ukrainian Journal of Ecology 11 (7), 154-168.
Received: 10-Aug-2021 Accepted: 22-Sep-2021 Published: 27-Sep-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
