Research - (2022) Volume 12, Issue 8
Formation of phytopathogenic complex Rosa in phytocenosis cultures with varietal variability of resistance indicators against Sphaerotheca pannosa var. rosae and Diplocarpon rosae
M. Alla Borysivna*, K.N. Mykolaiivna, M.V. Petrovych, O.O. Gennadiivna, R.S. Volodymyrovych and Z.K. ViacheslavivnaAbstract
A plant organism is the basis of urban ecosystem, the species diversity of flowering and ornamental plants is the formation factor of the microbiocenosis structure, its quantitative and qualitative composition. The aim of the work is to establish the influence of varietal variability of resistance indicators to Sphaerotheca pannosa var. rosae and Diplocarpon rosae on the Rosa genus plants for the formation of phytopathogenic complex in phytocenosis cultures.
To achieve this goal we performed the following tasks: to select Rosa cultivars for biocontrol of phytopathological complex formation in urbanized ecosystems on the basis of evaluation of immunological reaction of collection samples of these plants in natural infectious background.
Scientific novelty is determined by the following research results: there were studied ecological and biological bases of conducting phytomeliorative measures and selection of roses assortment for formation of resistant groups of flower-ornamental plants of phytocenosis cultures; as a result of immunological evaluation of roses varieties, there was determined a list of varieties that are the selection factors of highly virulent pathotypes of fungi Sphaerotheca pannosa Lev var. rosae Woron. and Diplocarpon rosae F.A.Wolf for biocontrol of phytopathogenic mycobiota.
During the years of research, a stable (As ≥ 60.1%) manifestation of the resistance indicator afainst Diplocarpon rosae was found in 46 cultivars (67.6%), and against Sphaerotheca pannosa var. rosae in 43 (63.3%)-collection cultivars of the Rosa genus. In regional collections of roses in terms of resistance against Sphaerotheca pannosa var. rosae, Diplocarpon rosae the samples were represented mainly by practically resistant forms (48 ± 5.2%). Practical significance for biocontrol of phytopathogens in urban ecosystems and as a source of resistance for assortment fitting and selection, for agroecology as the selection factor of highly virulent pathotypes of the fungus Sphaerotheca pannosa var. rosae have 20 cultivars of tea-hybrid roses, 12-of twisting roses, 3-of floribunda roses, 4-of English roses, as for Diplocarpon rosae-there are 27 varieties from the group of tea-hybrid roses, 4-twisting roses, 2-floribunda roses, 4-English roses.
Keywords
Urban ecosystem, Resistance, Rosa, Sphaerotheca pannosa var. rosae, Diplocarpon rosae.
Introduction
Constant growth of anthropogenic pressure on complex green areas of settlements, especially in large, medium-sized cities, which are economically developing and densely populated, contributes to scale and degree increase of transformation of the structural and functional organization of urban ecosystems. They are caused by a complex of changes occurring in special purpose plantations: damage to susceptible plant species, weakening and disruption of ecosystem connections, mycobiotic adaptations, invasion of pathogens into urban ecosystems, introduction of ornamental plants, changes in their resistance, productivity and role. Epiphytotics of mycoses on edificators lead to changes in the species floristic composition, structure and shape of the flower arrangement, consort connections and their functional properties, the decorative phytocenosis culture integrity is being damaged.
The use of varieties with different resistance in landscaping directly affects the nature of phytopathogen development. Under the same conditions of growth in the plantations of susceptible varieties, there may develop epiphytotics, and with a resistant one-there is a slow disease development or no pathology. The resistance genotype generates significant selective pressure on the fungus population, which leads to selective preservation and rapid reproduction of virulent pathotypes. Continuous variability of parasitic properties causes loss of variety resistance. That is, the biotic factors influence composition and population dynamics of phytopathogens, among which the leading place is occupied by the selective pressure of resistant varieties. A plant organism is the basis of the urban ecosystem, the species diversity of flowering and ornamental plants is the formation factor of the microbiocenosis structure, its quantitative and qualitative composition.
It should also be noted that the varieties’ resistance is a short-term phenomenon, because during their cultivation appear new types of phytopathogenic microorganisms or their frequency increases, which weakens the existing resistance. The rate, at which this occurs, depends not only on the phytopathogen variability, but also on the host plant resistance mechanism. Varieties that have lost their resistance become reservoirs of highly pathogenic races and strains of phytopathogenic mycobiota, which in the process of reproduction can cause epiphytotics. Wide specialized necrotrophic fungi species are considered as particularly dangerous, which are able to parasitize on a large number of cultivated plants species, get quickly accumulated and be stored on seeds, fruits, plant remains and in soil for a long time. Therefore, a resistant variety, especially created by genetic modification, is a powerful factor in targeted selection in populations of microorganisms by the pathogenicity and aggression indicators, and a susceptible variety is a growth factor of their populations. They significantly affect the qualitative and quantitative indicators of the phytopathogenic complex, which significantly worsens the conditions of phytocenosis cultures and to some extent the biological resistance of urban ecosystems.
The Rosa genus includes about 400 species (Khrzhanovskyi, 1958), most of which are resistant to diseases and pests, and the world assortment of roses, created on the basis of boreal and subtropical species, now has about 40 thousand varieties and forms, combined in 39 garden groups (Modern Roses, 2007). During the last 200 years, the selection of garden roses was aimed mainly at improving the decorative features and inter-varietal crosses were used more frequently for hybridization, as a result of which most roses’ varieties lost immunity to diseases, inherent in wild species (Klymenko, 2009). Information on the resistance of the Rosa genus plants against diseases is reflected in the works of (Baumane, 1979, Bondarenko-Borysova, 2008, Golovchenko, 2007, Gorlanova, 2013. Gorlanova, Tereshkin, 2014. Gorlenko, Panko, 1984. Kachurina, 1958. Klymenko, 2008. Mandre, 1971. Rieksta, 1971. Ruzaeva, 2007).
The work aim is to establish the influence of varietal variability of resistance indicators against Sphaerotheca pannosa var. rosae and Diplocarpon rosae, using plants of the Rosa genus, on the formation of phytopathogenic complex in phytocenosis cultures.
To achieve this goal we performed the following tasks:
To select Rosa cultivars for biocontrol of phytopathological complex formation in urbanized ecosystems on the basis of evaluation of immunological reaction of collection samples of these plants in natural infectious background.
Scientific novelty is determined by the following theoretical and practical research results:
Ecological and biological bases for conducting phytomeliorative measures and selection of roses assortment for formation of resistant groups of flower-ornamental plants of phytocenosis cultures;
As a result of immunological evaluation of roses cultivars, there was determined a list of varieties that are the selection factors of highly virulent fungi pathotypes Sphaerotheca pannosa Lev. var. rosae Woron. and Diplocarpon rosae F.A.Wolf for biocontrol of phytopathogenic mycobiota.
Materials and Methods
Immunological evaluation of Rosa collection varieties in natural infectious background against Sphaerotheca pannosa var. rosae, Diplocarpon rosae was performed on 69 varieties from 4 groups (tea-hybrid roses, twisting roses, English roses, floribunda roses).
Determination of the collection material resistance against diseases was performed by methods of plant damage visual assessment in natural background during vegetation in the plant ontogenesis dynamics by a score scale. Observations were performed on plants of different ages during the period of maximum development of pathologies (July-August-September), using a 9-point scale (Kulibaba, Primakovskaya, 1974. Babayants et al., 1988). Characteristics of the resistance level of the Rosa collection cultivars was performed by scales, shown in Table 1.
| Calculation scale | Resistance characteristics by: | |||
|---|---|---|---|---|
| Score | % | Score | Reaction type | Degree |
| 0 | 0 | 9 | resistance (R) | immune І (+3σх) |
| 1.0 | 0.1-15.0 | 7 | moderately resistance (R+) | practically resistant ІІ (+2σх) |
| 2.0 | 15.1-35.0 | 5 | moderately susceptible (S/) | moderately resistant ІІІ (±σх) |
| 3.0 | 35.1-50.0 | 3 | susceptible (S) | susceptible IV (-2σх) |
| 4,0 | >50.1 | 1 | highly susceptible (S+) | highly susceptible V (-3σх) |
Table 1. Scale for assessing the resistance level by VIR and CMEA scale (Shkalikov et al.; 2005).
The coefficient of variation is a relative quantity used to characterize the fluctuations (variability) of an indicator. The quadratic coefficient of variation is the percentage of the mean quadratic deviation to the mean level, was calculated by the formula:
Vσ=Q/X × 100,
Where Vσ is the coefficient of variation; Q is the mean quadratic deviation; X is the mean size of the indicator in the statistical cumulation. The smaller this figure, the smaller is the fluctuations of the indicator in the cumulation and the more homogeneous is the cumulation, and vice versa. The quadratic coefficient of variation was established by a scale: Vσ ≤ 33.0%-the cumulation is homogeneous; Vσ ≤ 40%-the indicator fluctuates slightly; Vσ ≤ 60%-the indicator fluctuates moderately; Vσ>60%-the indicator fluctuates significantly.
Thus, their stability level characteristics were determined by the alignment coefficient (B,%), which gradations are given in the work of V.S. Gorya (Gorya, 1978).
В=100-Vσ,
Where Vσ parameter is the coefficient of variation. Thus, for B over 90%-the alignment of the indicator is high, from 80 to 90%-average; less than 80%-low.
The coefficient of stability of the resistance indicator was determined by V.V. Khangildin. (Khangildin, Litvinenko, 1981). by the formula:
Аs=100-(S/Х),
where S is the standard deviation; X-the average annual degree of damage; As-is the coefficient of the manifestation stability of the varietal resistance indicator,%.
According to the results of long-term evaluations, the samples were classified into five groups of resistance according to the following scale, in points or percentages of the average annual damage: immune; practically resistant (Вх=0.1-1.0; х=0.1-25%); lowly susceptible (Bx=2.1-3.0; x=25.1-50.0%); moderately susceptible (Bx=2.1-3.0; x=50.1-75.0%); susceptible (Bx>3.1; x>75.1%). The final analysis of the level and stability was performed using the damage indicators Lim Xmax, coefficient of agronomic stability and indices of the resistance level in accordance with the generalized scale: highly resistant-no signs of damage; practically resistant (Lim Xmax<25,0%; As> 60,0%, index 9 and 7); lowly susceptible (Lim Xmax<25.1-37.5%; As>60.1%, index 9,7 and 5); susceptible (Lim Xmax<25.1-37.5%; As>40.0%, index 9 and 7); moderately susceptible (Lim Xmax<37.6-63.5%; As>40.0%, index 9, 7 and 5).
Stable practical resistance or susceptibility is characterized by index 9 and 7, and conditional-5, 3 and 1. The level of resistance stability or susceptibility reflects the index, according to the scale:
9-very high level of stability of the resistance indicator (As>80.1%);
7-high (As=60.1-80.0%);
5-average (As=40.1-60.0%);
3-low (As=20.1-40.0%);
1-very low (As <20.0%).
Stable manifestation of the resistance indicator is characterized by index 9 and 7, and conditional-5, 3 and 1. The heterogeneity of samples with availability of practically resistant genotypes and the pattern of their distribution in populations by the degree (level) of resistance indicator alignment was determined by classical statistical methods (Wolf, 1966). Derkach, 1963. Dospekhov, 1968).
Results
As a result of phytopathological monitoring of the Rosa genus collection cultivars, it was established that the group of tea-hybrid roses had average annual damage rates by powdery mildew Sphaerotheca pannosa var. rosae 11.6% within Lim Xmin-max 0.0-36.07% with average score of 0.8 Lim Bxmin-max 0.0-2.18, twisting roses-8.5% (0.0-34.3) and 0.86 (0.0-1.8), English roses-2.3% (0.0-15.7) and 0.8 (0.0-1.15), floribunda roses-6.3 % (0.0-7.0) and 0.52 (0.0-0.86), respectively (Table 2).
| Cultivar group | Variability Indicators Symbols | |
|---|---|---|
| Lim Хmin-max, % | Lim Вхmin-max | |
| Sphaerotheca pannosa var. rosae | ||
| Tea-hybrid roses | 0.0-36.07 | 0.0-2.18 |
| Twisting roses | 0.0-34.3 | 0.0-1.8 |
| Floribunda roses | 0.0-7.0 | 0.0-0.86 |
| English roses | 0.0-15.7 | 0.0-1.15 |
| Diplocarpon rosae | ||
| Tea-hybrid roses | 0.0-30.7 | 0.0-1.8 |
| Twisting roses | 0.0-28.5 | 0.0-1.8 |
| Floribunda roses | 11.0-17.7 | 0.7-1.0 |
| English roses | 0.0-15.4 | 0.0-0.8 |
Table 2. Damage variability by powdery mildew and black spot of the Rosa genus cultivars leaves in the natural background.
As a result of phytopathological monitoring of the Rosa genus collection cultivars, it was found that the group of tea-hybrid roses had average annual damage rate of leaves by black spot Diplocarpon rosae 8,5% within Lim Xmin-max 0.0-30.7% with an average score of 0.7 within Lim Bxmin-max 0.0-1.8, twisting roses-14.2% (0.0-28.5) and 0.8 (0.0-1.8), English roses-8.73%, 0-15.4) and 0.6 (0.0-0.8), floribunda roses-14.1% (11.0-17.7) and 0.8 (0.7-1.0), respectively (Table 2).
According to the results of immunological evaluation of the Rosa genus collection cultivars cumulation within the damage by D. rosae and Sp. pannosa var. rosae in the natural background by a record scale, it was found that division is being done into four groups (Fig. 1). The immune group (R) in terms of resistance to D. rosae includes 22.1% of collection cultivars, and to Sp. pannosa var. rosae-25%, practically resistant (R+)-50 and 48.5%, medium resistant (S/)-26.7 and 20.6%, susceptible (S)-1.2 and 5.9%, respectively, at the same time-very susceptible (S+) was not detected.
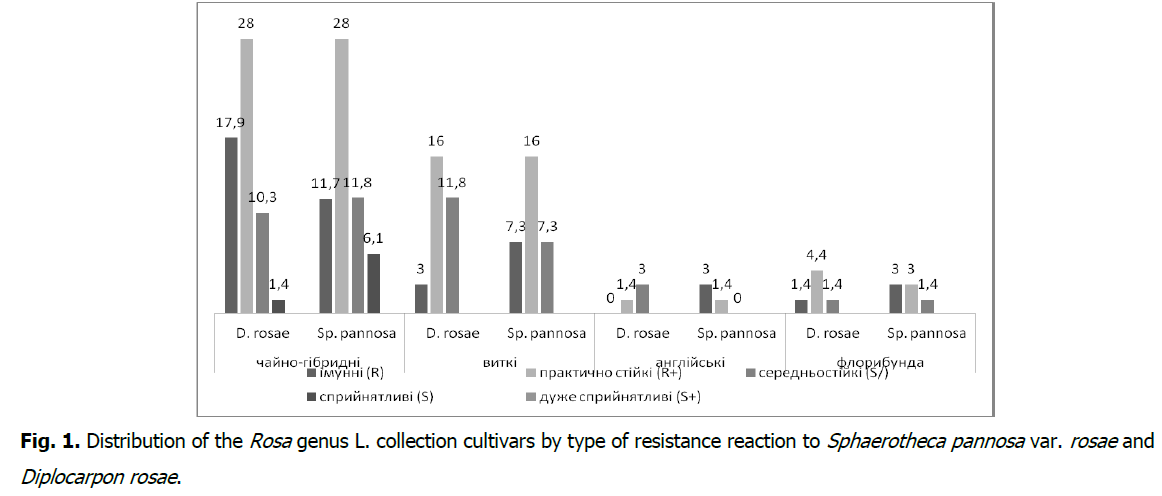
Fig 1: Distribution of the Rosa genus L. collection cultivars by type of resistance reaction to Sphaerotheca pannosa var. rosae and Diplocarpon rosae.
According to the rose leaves resistance manifestation to black spotting and powdery mildew in terms of groups, the distribution is as follows: tea-hybrid roses are divided into immune (R) against D. rosae-17.9% of collection varieties, and for Sp. pannosa var. rosae-11,7%, practically resistant (R+)-28% each, medium resistant (S/)-10.3 and 11.8%, susceptible (S)-1.4 and 6.1%; twisting roses-immune (R)-3.0 and 7.3%, almost resistant (R+) 16%, moderately resistant (S/)-11.8 and 7.3%; English roses-immune (R)-1,4 and 3.0%, almost resistant (R+)-4.4 and 3.0%, moderately resistant (S/)-1.4%; floribunda roses-immune (R)-0 and 3.0%, almost resistant (R+)-1.4%, medium resistant (S/)-3.0 and 0.0%, to the total number of studied cultivars, respectively (Fig. 1).
In the collection cultivars of the Rosa genus, a stable (As ≥ 60.1%) manifestation of the resistance indicator to Diplocarpon rosae was found in 46 cultivars (67.6%) (Fig. 2), and to Sphaerotheca pannosa var. rosae in 43 (63.3%) cultivars (Fig. 3). It should be noted that the resistance group I-immune with the type of reaction (R) against Diplocarpon rosae is represented by 15 cultivars (2.0%), and against Sphaerotheca pannosa var. rosae-by17 (25.0%) cultivars with a very high level of stability of the resistance indicator (As>80.1%).
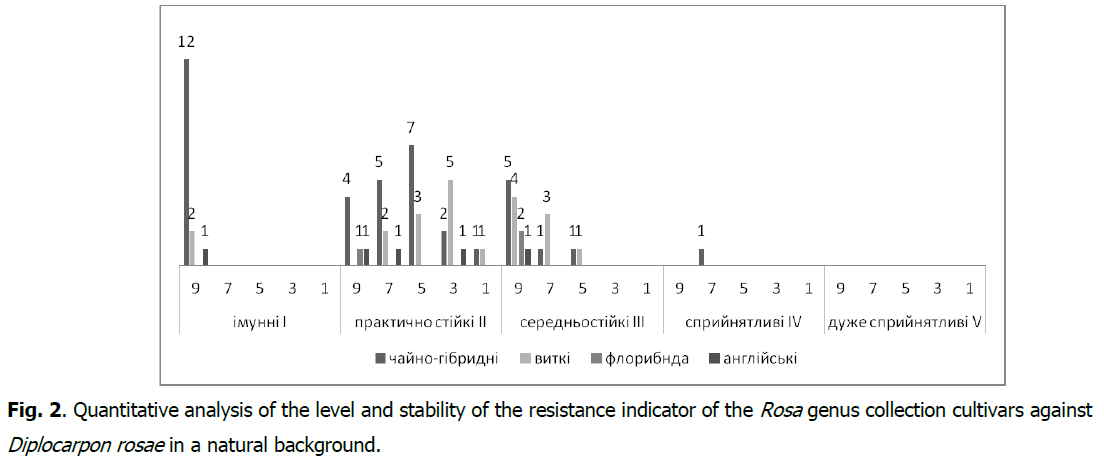
Fig 2: Quantitative analysis of the level and stability of the resistance indicator of the Rosa genus collection cultivars against Diplocarpon rosae in a natural background.
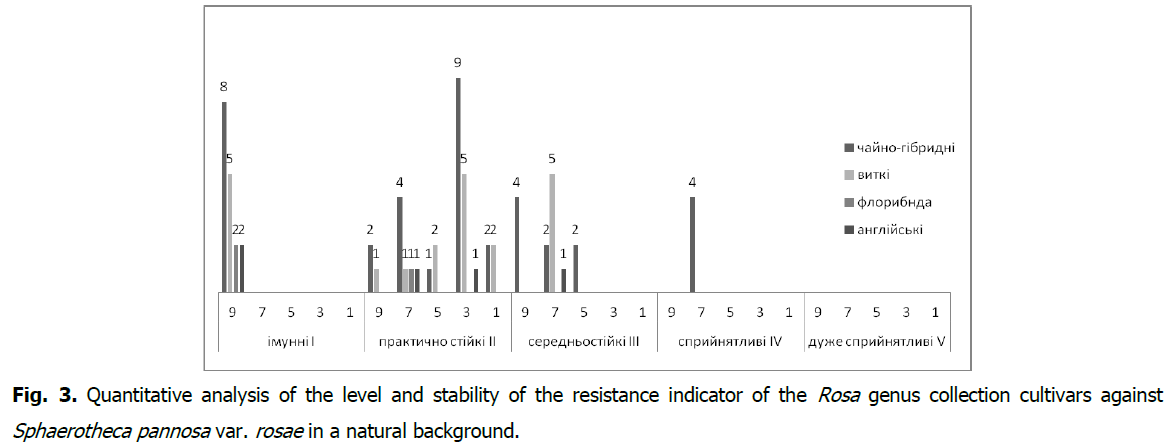
Fig 3: Quantitative analysis of the level and stability of the resistance indicator of the Rosa genus collection cultivars against Sphaerotheca pannosa var. rosae in a natural background.
First of all, our research was aimed at selecting stable sources from the collection of samples and creating on them a database for further selection work with the lowest possible degree of damage to plants of the Rosa genus by black spot and powdery mildew in a natural infectious background.
Based on the obtained experimental data, we note that in the natural background there is a minimum degree of damage (score 9), but with a fairly high coefficient of population uniformity of this indicator (Vσ ≤ 33.0%; B ≥ 90) relative to D. rosae was found in 62% populations of rose cultivars and against Sp. pannosa var. rosae in 60%. In the natural background, the rose collection samples population consists of homogeneous by leaves resistance to powdery mildew and black spot in the group of tea-hybrid roses by 31.9 and 41.2%, twisting roses-by 14.5%, floribunda roses-by 3 and 4.4%, English roses-by 5.8 and 4.4%, respectively, of the total sample number among the research subjects.
The amplitude of roses damage variability by the pathogen Sphaerotheca pannosa var. rosae over the years of research proves that the homogeneous are: in the group of tea-hybrid roses 55% of the cultivars population, of the total number of samples in the group: 'Black Lady', 'Black Baccara', 'Rose Gaujard', 'Russkaya krasavitsa', 'Zolotoi Jubilees ',' Paradise Weeks ',' Blue River ',' Flamingo ',' Pink Paradise ',' Janina ',' Lady Rose ',' Monarch ',' Norita ',' Piroschka ',' Red Magic ',' Red Star ',' Rose Giardinodi Boboli ',' Valentino ',' White Romance ',' Blue Parfum ',' Emmy '(Table 3); in the group of twisting roses-47,6% of the cultivars population: 'American Pillar', 'Coral Dawn', 'Elegance', 'Excelsa', 'Sedaja Dama', 'Super Hero', 'Wartburg', 'New Dawn', 'Flammentanz ',' Pierre de Ronsard ' (Table 4); in the floribunda roses group-66,6% of the cultivars population: ‘Leonardo da Vinci’, ‘Aspirin’; in the English roses group-80,0%: ‘Abraham Darby’, ‘Golden Celebration’, ‘Princess Alexandra of Kent’, ‘William Shakespeare’ (Table 5).
| Cultivar | Indicator | Immunologic evaluation | ||||
|---|---|---|---|---|---|---|
| Lim Х min-max, % | Lim Вх min-max | Аs | Resistance level index | Reaction type | level | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Sphaerotheca pannosa var. rosae | ||||||
| ‘Black Lady’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Black Baccara’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Flamingo’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Rose Gaujard’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Russkayakrasavitsa’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘ZolotoiYubile’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Paradise Weeks’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Blue River’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Emmy’ | 10-16 | 0.64-1.0 | 81.2 | 9 | (R+) | ІІ (+2σх) |
| ‘Monarch’ | 10-15 | 0.64-1.0 | 85.8 | 9 | (R+) | ІІ (+2σх) |
| ‘Pink Paradise’ | 10-15 | 0.64-2.0 | 74,5 | 7 | (R+) | ІІ (+2σх) |
| ‘Blue Parfum’ | 5-12 | 0.64-1.0 | 69.2 | 7 | (R+) | ІІ (+2σх) |
| ‘Janina’ | 4-10 | 0.64-1.0 | 67.2 | 7 | (R+) | ІІ (+2σх) |
| ‘Nostalgie’ | 4-10 | 0.64-1.0 | 63.3 | 7 | (R+) | ІІ (+2σх) |
| Diplocarpon rosae | ||||||
| ‘Alexander’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Dame de Coeur’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Glorious’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Pink Paradise’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Julia’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Prima Ballerina’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Red Magic’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Red Star’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘ZolotoiYubilei’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Blue River’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Flamingo’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Black Magic’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Imperatrice Farah’ | 10-14 | 0.6-1.0 | 81.6 | 9 | (R+) | ІІ (+2σх) |
| ‘Black Baccara’ | 4-7 | 0.4-1.0 | 81.7 | 9 | (R+) | ІІ (+2σх) |
| ‘Rose Gaujard’ | 6-10 | 0.25-1 | 82.1 | 9 | (R+) | ІІ (+2σх) |
| ‘Princesse de Monaco’ | 12-15 | 0.8-1.0 | 91.9 | 9 | (R+) | ІІ (+2σх) |
| ‘Blue Parfum’ | 18-35 | 1.5-2.0 | 70 | 7 | (R+) | ІІ (+2σх) |
| ‘Rose Giardino di Boboli’ | 3-9 | 0.25-1 | 67.2 | 7 | (R+) | ІІ (+2σх) |
| ‘Janina’ | 5-12 | 0.5-1.0 | 75.7 | 7 | (R+) | ІІ (+2σх) |
| ‘Augusta Luise’ | 6-12 | 0.25-1.0 | 74 | 7 | (R+) | ІІ (+2σх) |
| ‘Peace’ | 7-15 | 0.2-1.0 | 65.4 | 7 | (R+) | ІІ (+2σх) |
Table 3. Variety of cultivars collection of the Rosa genus group of tea-hybrid roses with leaves stable resistance indicator to powdery mildew and black spotting in a natural background.
| Cultivar | Indicator | Immunologic Evaluation | ||||
|---|---|---|---|---|---|---|
| Lim Хmin-max, % | Lim Вхmin-max | Аs | Resistance Level Index | Reaction type | Level | |
| Sphaerotheca pannosa var. rosae | ||||||
| ‘SedajaDama’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Super Hero’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Wartburg’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘New Dawn’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Flammentanz’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Excelsa’ | 0-10 | 0-0.8 | 92.7 | 9 | (R+) | ІІ (+2σх) |
| ‘Amethyste’ | 5-12 | 0.64-1.0 | 65.2 | 7 | (R+) | ІІ (+2σх) |
| Diplocarpon rosae | ||||||
| ‘Coral Dawn’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘KrasnyjMajak’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Elegance’ | 0-7 | 0.0-0.3 | 61.8 | 7 | (R+) | ІІ (+2σх) |
| ‘KrymskijeZori’ | 0-15 | 0.0-1.0 | 54.8 | 7 | (R+) | ІІ (+2σх) |
Table 4. Variety of collection cultivars of the Rosa genus group of twisting roses with a stable resistance manifestation indicator against powdery mildew and black spot of leaves in a natural background.
| Cultivar | Indicator | Immunologic Evaluation | ||||
|---|---|---|---|---|---|---|
| Lim Хmin-max, % | Lim Вхmin-max | Аs | Resistance level index | Reaction type | level | |
| Diplocarpon rosae | ||||||
| Floribunda | ||||||
| ‘Aspirin Rose’ | (8-13)9 | 0.5-1.0 | 81.8 | 9 | (R+) | ІІ (+2σх) |
| English Rose | ||||||
| ‘Princess Alexandra of Kent’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘William Shakespeare’ | (10-15)9 | 0.64-1.0 | 81.9 | 9 | (R+) | ІІ (+2σх) |
| ‘Golden Celebration’ | (5-17)7 | 0.5-1.2 | 61.3 | 7 | (R+) | ІІ (+2σх) |
| Sphaerotheca pannosa var. rosae | ||||||
| Floribunda | ||||||
| ‘Leonardo da Vinci’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Aspirin Rose’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Jubile du Prince de Monaco’ | 5-9 | 0.64-1.0 | 74.3 | 7 | (R+) | ІІ (+2σх) |
| English Rose | ||||||
| ‘Abraham Darby’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Golden Celebration’ | 0.0 | 0.0 | 100 | 9 | (R) | І (+3σх) |
| ‘Princess Alexandra of Kent’ | 5-9 | 0.64-1.0 | 74.3 | 7 | (R+) | ІІ (+2σх) |
Table 5. Variety of collection cultivars of the Rosa genus group of floribunda group and English roses with a stable resistance manifestation indicator against powdery mildew and black spot of leaves in a natural background.
The amplitude of roses damage variability by the pathogen Diplocarpon rosae over the years of research proves that the homogeneous are: in the group of tea-hybrids 70% of the cultivars population: 'Zolotoi Yubilei', 'Alexander', 'Dame de Coeur', 'Glorious', 'Julia', 'Prima Ballerina', 'Red Magic', 'Red Star', 'Pink Paradise', 'Blue River', 'Flamingo', 'Black Magic', 'Black Baccara', 'Imperatrice Farah', 'Janina', 'Karen Blixen ',' Kardinal 85 ',' Krymskaja noth ',' Princesse de Monaco ',' Rose Gaujard ',' Rose Giardinodi Boboli ',' Valentino ',' Victor Borge ',' Paradise ',' Pink Intuition ',' Blue Parfum ',' Augusta Luise ' (Table 3); in the group of twisting roses-47,6%: 'American Pillar', 'Amethyste', 'Belosnezhka', 'Coral Dawn', 'Dorothy Perkins', 'Krasnyj Majak', 'New Dawn', 'Polka', 'Flammentanz', ‘Pierrede Ronsard’ (Table 4); in the English roses group-60,0%: ‘Abraham Darby’, ‘William Shakespeare’, ‘Princess Alexandra of Kent’ (Table 5).
During the years of research in a natural background, there was found no manifestation of powdery mildew Sp. pannosa var. rosae on cultivars: in the group of tea-hybrid roses-'Black Lady', 'Black Baccara', 'Rose Gaujard', 'Russkaya krasavitsa', 'Zolotoi Yubilei', 'Paradise Weeks', 'Blue River', 'Flamingo' (Table 3); twisting roses-‘Sedaja Dama’, ‘Super Hero’, ‘Wartburg’, ‘New Dawn’, ‘Flammentanz’ (Table 4); floribunda roses-‘Leonardo da Vinci’, ‘Aspirin Rose’; English-‘Abraham Darby’, ‘Golden Celebration’ (Table 5).
During the years of research in a natural background, there was found no manifestation of leaves black spotting of Diplocarpon rosae on cultivars: in the group of tea-hybrid roses-'Alexander', 'Dame de Coeur', 'Glorious',' Julia ',' Prima Ballerina ',' Red Magic ',' Red Star ',' Zolotoi Yubilei ',' Pink Paradise ',' Blue River ',' Flamingo ','Black Magic' (Table 3); twisting roses-‘Krasnyj Majak’, ‘Coral Dawn’ (Table 4); English roses-‘Princess Alexandra of Kent’ (Table 5).
Resistance group II-practically resistant with reaction type (R +) against Diplocarpon rosae and Sphaerotheca pannosa var. rosae is represented by cultivars in the group of tea-hybrid roses-26,5 and 27,9%, coils-52,4 and 53,4%, respectively, floribunda-33,4%, English roses-40,0%.
Very high (As> 80,1%) and high (As=60,1-80,0%) level of practical resistance indicator against Diplocarpon rosae have samples from group of tea-hybrid roses: 'Black Baccara', 'Imperatrice Farah', ' Princesse de Monaco ',' Rose Gaujard 'and' Augusta Luise ',' Blue Parfum ',' Rose Giardino di Boboli ',' Janina ',' Peace ', respectively (Table 3); from the group of twisting roses: ‘Krymskije Zori’, ‘Elegance’ have high indicator level (Table 4); English roses: ‘William Shakespeare’ and ‘Jubilee Celebration’, respectively, floribunda roses: ‘Aspirin Rose’ has very high indicator level (Table 5).
A very high indicator level (As> 80,1%) of practical resistance to Sp. pannosa var. rosae have samples from the tea-hybrid group: 'Emmy', 'Monarch', twisting roses: 'Excelsa' and high (As=60,1-80,0%) from the tea-hybrid group: 'Nostalgie', 'Blue Parfum ',' Pink Paradise ',' Janina ' (Table 3); twisting roses: ‘Amethyste’ (Table 4); floribunda roses: ‘Jubiledu Prince de Monaco’, English roses: ‘Princess Alexandra of Kent’ (Table 5).
Conclusion
A result of immunological evaluation of roses cultivars, there was determined a list of varieties that were factors in the selection of highly virulent pathotypes of fungi Sphaerotheca pannosa Lev. var. rosae Woron. and Diplocarpon rosae F.A.Wolf for biocontrol of phytopathogenic mycobiota.
Stable (As ≥ 60.1%) manifestation of the resistance indicator against Diplocarpon rosae was found in 46 cultivars (67.6%), and against Sphaerotheca pannosa var. rosae in 43 (63.3%)-collection cultivars of the Rosa genus.
Practical significance for selection as a source of resistance and for agroecology as a selection factor of highly virulent pathotypes of the fungus Sphaerotheca pannosa var. rosae have such cultivars: group of tea-hybrid roses: 'Black Lady', 'Black Baccara', 'Rose Gaujard', 'Russkaya krasavitsa', 'Zolotoi Yubilei', 'Paradise', 'Blue River', 'Flamingo', 'Monarch ','Emmy ','Janina ','Pink Paradise ','Blue Parfum ','Nostalgie','Norita','Piroschka','Red Magic','White Romance','Princesse de Monaco', 'Red Star'; group of twisting roses: 'Sedaja Dama', 'Super Hero', 'Wartburg', 'New Dawn', 'Flammentanz', 'Excelsa', 'Amethyste', 'American Pillar', 'Belosnezhka', 'Coral Dawn', 'Elegance',' Pierre de Ronsard '; group of floribunda roses: ‘Leonardo da Vinci’, ‘Aspirin Rose’, ‘Jubiledu Prince de Monaco’; group of English roses: ‘Abraham Darby’, ‘Golden Celebration’, ‘Princess Alexandra ofKent’,‘William Shakespeare’.
Intensive development of both high-and low-virulence pathogens that lead to the appearance of powdery mildew epiphytoses, which increase the rate of formation and emergence of Sphaerotheca pannosa var. rosae aggressive races, are promoted by cultivars from the tea-hybrids group: 'Julia', 'Alexander', 'Dame de Coeur', 'Imperatrice Farah', 'Karen Blixen', 'Kardinal 85', 'Krymskaja noth', 'Roter Stern', 'Victor Borge','Augusta Luise','Black Magic ','Peace ','Double Delight','Glorious', 'Prima Ballerina', 'Lady Rose', 'Rose Giardinodi Boboli', 'Valentino', 'Pink Intuition', 'Red Star'; twisting roses: ‘New dreams’, ‘Polka Babochka’, ‘Dorothy Perkins’,‘Devich’ji Grezy’, ‘Krasnyj Majak’,‘Krymskije Zori’, ‘Polka’, ‘New Dawn’, ‘Rosarium Uetersen’; English roses: ‘Jubilee Celebration’.
Practical significance for selection as a source of resistance and for agroecology as a selection factor of highly virulent pathotypes of the fungus Diplocarpon rosae have cultivars of tea-hybrid roses group: 'Alexander', 'Dame de Coeur', 'Glorious', 'Julia', 'Prima Ballerina', 'Red Magic', 'Red Star', 'Zolotoi Yubilei', 'Pink Paradise', 'Blue River', 'Flamingo', 'Black Magic', 'Black Baccara', 'Imperatrice Farah', 'Princesse de Monaco', 'Rose Gaujard', 'Janina', 'Rose Giardinodi Boboli', 'Augusta Luise', 'Peace', 'Karen Blixen', 'Kardinal 85', 'Valentino', 'Victor Borge', 'Paradise', 'Krymskaja noth ',' Pink Intuition','Blue Parfum'; twisting roses: ‘Coral Dawn’, ‘Krasnyj Majak’, ‘Elegance’, ‘Krymskije Zori’; floribunda roses: ‘Leonardo da Vinci’, ‘Jubiledu Prince de Monaco’; English roses: ‘Princess Alexandra of Kent’, ‘William Shakespeare’; ‘Abraham Darby’, ‘Golden Celebration’.
Intensive development of both high-and low-virulence pathogens Diplocarpon rosae, leading to epiphytosis of black spot on leaves, which increase the rate of formation and emergence of aggressive races is contributed by the cultivar groups like: tea-hybrid roses: 'Black Lady', 'Lady Rose', 'Monarch', 'Norita', 'Russkaya krasavitsa', 'White Romance', 'Nostalgie', 'Piroschka', 'Emmy', 'Double Delight', 'Roter Stern'; twisting roses: 'Super Hero', 'Wartburg', 'New Dawn', 'Excelsa', 'Newdreams',' Polka Babochka ',' Rosarium Uetersen ',' Sedaja Dama ',' Devich'ji Grezy','New Dawn '; English roses: ‘Jubilee Celebration’.
References
Baumane, G.K. (1979). Some biochemical aspects of rose resistance against powdery mildew. Wealth of flora-for the national economy: materials of the conference "Problems of Study and Use of Natural Flora Plants in the National Economy." Moscow: SBG AS SSSR, pp:367-370.
Bondarenko-Borisova, I.V. (2008). Diseases of the garden hybrid rose (Rosa × hybrida hort.) in the donetsk botanical garden collection of the Ukraine National Academy of Sciences and methods of their control. Industrial Botany, 8:240-249.
Wolf, W.G. (1966). Statistical processing of experimental data. Moscow: Kolos, p:253.
Golovchenko, L.A. (2007). Morphological and cultural properties of Botrytis cinerea Pers. isolates, pathogens of rose gray rot. Theoretical and applied aspects of plant introduction as a promising direction of science and national economy development: materials of the International scientific conference dedicated to the 75th anniversary of the CBGNAS of Belarus. NAS of Belarus, CBG. Minsk: Edit VV, 2:203-205.
Gorlanova, E.P. (2013). Diseases of Rosa hybrid hort. in the Lower Volga region and measures to combat them. Bulletin of the Botanical Garden of Saratov State University. Saratov: Saratov University Publishing House, 11:244.
Gorlanova, E.P., Tereshkin, A.V. (2014). Black spotting of roses and measures to combat it in the lower Volga region. Agrarian Scientific Journal. Natural Sciences, 10:6-8.
Gorlenko, S.V., Panko, N.A., Podobnaya, N.A. (1984). Pests and diseases of a rose. Minsk: Nauka i tehnika, p:128.
Gorya, V.S. (1978). Algorithms for mathematical processing of research results. Chisinau: Shtiintsa, pp:21-23.
Derkach, M.P. (1963). Elements of statistical processing of the results of a biological experiment. Lviv: Lviv University Press, p:67.
Armor B.A. (1968). Field experience methodology. Moscow: Kolos, p:336.
Zaitsev, G.N. (1984). The mathematical statistics in experimental botany. The Mathematical Statistics in Experimental Botany.
Shkalikov, V.A. (2005). Plant immunity. Moscow: Kolos, p:190.
Kachurina, L.I. (1958). Genus rosa L. rose (dog rose). Ornamental plants for the Far North of the USSR. Moscow-Leningrad: Publishing House of the USSR Academy of Sciences, p:13-17.
Marchenko, A.B. (2015). Phytopathogenic complex of pathogens of ornamental shrubs of the genus Rosa L. Hortus Botanicus.
Klimenko, Z.K. (2008). The work results of many years (1812–2008) on the introduction of garden roses in the Nikitskyi Botanical Garden. Papers of Nikitsky Botanical Garden, 130:68-75.
Litun, P.P. (1980). Genotype-environment interaction in genetic and selection studies and evaluation of selection material. Kiev: Naukova Dumka, p:63-93.
Mandre, M. (1971). Biochemical characteristics of roses affected by powdery mildew. Baltic Botanical Gardens. Riga: Zinatne, p:209-215.
Kulibaba, Y.F., Primakovskaya, M.A. (1974). Methodical instructions for detection and accounting of flower cultures diseases. Moscow: Kolos, p:19-26.
Dyutin, K.E. (1981). Methodical instructions for resistance assessing of melon crops against fusarium wilt. Moscow, p:12.
Babayants, L.T. (1988). Methods of selection and assessment of wheat and barley resistance in CMEA member countries. Prague, p:125-295.
Minkevich, I.I., Zakharova, T.I. (1977). Mathematical methods in phytopathology. Leningrad: Kolos, p:8-15.
Rieksta, D.A. (1971). Variety study and selection of roses in the Latvian SSR: Thesis Abstract of Agricultural Science and Tallinn, p:26.
Ruzaeva, I.V. (2007). Resistance of garden roses against diseases. Samarskaya Luka: Bull. 16:91-109.
Hangildin, V.V., Litvinenko, N.A. (1981). Homeostatics and adaptability of winter wheat varieties. Scientific and Technical Bul. AAI. Odessa, 39:8-14.
Khrzhanovskyi, V.I. (1958). Roses. Moscow: Sov Nauka, p:497.
Chernenko, K.M. (2003). Peculiarities of parasitism of black rot pathogens and carrot selection source material for resistance. Thesis Abstract of Biology Science and Kharkiv, p:35.
Chumakov, F.U., Minkevich, I.I. (1974). Basic methods of phytopathological research. Moscow: Kolos, p:407.
Young, M.A., Schorr, Ph. (2007). Modern roses12: the comprehensive list of roses in cultivation or of historical or botanical importance. Shreveport: The American Rose Society, p:576.
Author Info
M. Alla Borysivna*, K.N. Mykolaiivna, M.V. Petrovych, O.O. Gennadiivna, R.S. Volodymyrovych and Z.K. ViacheslavivnaCitation: Alla Borysivna, M., Mykolaiivna, K.N., Petrovych, M.V., Gennadiivna, O.O., Volodymyrovych, R.S., Viacheslavivna, Z.K. (2022). Formation of phytopathogenic complex Rosa in phytocenosis cultures with varietal variability of resistance indicators against Sphaerotheca pannosa var. rosae and Diplocarpon rosae. Ukrainian Journal of Ecology. 12:1-11.
Received: 11-Jan-2021, Manuscript No. UJE-21-24447; , Pre QC No. P-24447; Editor assigned: 13-Jan-2022, Pre QC No. P-24447; Reviewed: 25-Jan-2022, QC No. Q-24447; Revised: 23-Sep-2022, Manuscript No. R-24447; Published: 30-Sep-2022, DOI: 10.15421/2022_393
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
