Review - (2023) Volume 13, Issue 6
Harnessing and sounding the alarm on Fusarium head blight of wheat: current status, biology, detection and diagnosis method, mycotoxins, and integrated management options
G.H. Regasa*Abstract
Wheat production is expected to increase despite increased global food demand due to the influence of population growth and climate change. Providing enough safe and ensuring sustainable wheat production for a rapidly growing world population poses many challenges. Among the most serious hazardous, Fusarium Head Blight (FHB) caused by fungi of the genus, Fusarium is one of the most dangerous and catastrophic wheat diseases. It has a wide geographic distribution and causes severe economic losses in wheat production worldwide. Several investigations noted that FHB epidemics were becoming widespread. An increasing frequency of FHB epidemics in wheat has resulted in significant yield reductions, and it is crucial to emphasize the future challenge of safeguarding wheat production under upcoming imminent climate change affecting environmental conditions. Climate change is aggravating FHB epidemics by increasing wheat stresses and expanding the natural ranges for Fusarium species. Multiple outbreaks of FHB have affected Ethiopian wheat producers over the last few years, most notably in the 2022 cropping season. The infection leads to mycotoxin accumulation in grains, jeopardizing its suitability for human and animal consumption. Moreover, due to the extreme toxicity of Fusarium mycotoxins and the impact of FHB on wheat production, prevention and control practices such as cultural practices, resistant cultivars and fungicide application must be integrated into the management strategy. Nowadays, there is an urgent need to make wheat production more robust and sustainable while still continuing to develop high-yielding, disease resistant and climate-smart wheat varieties. This review aims to provide an overview of pathogen biology, current status, detection method and integrated management strategies. Generally, to safeguard wheat production and productivity from the deadliest FHB, we must struggle and fight by all means open to science.
Keywords
Epidemics, Fusarium head blight, Integrated management, Mycotoxins, Wheat.
Introduction
Wheat (Triticum spp.) is the second most cultivated cereal crop globally next to rice with a production of 788.26 million metric tonnes from 220.30 million hectares of land having an average yield productivity of 3.58 tonnes per hectare (USDA, 2023). In Ethiopia, it is cultivated on a total area of 2.1 million hectares (1.7 million hectares rain-fed and 0.4 million hectares irrigated) annually with a total production of 6.7 million tonnes with an average productivity of 3.0 tonnes per hectare under rain-fed conditions during 2021/22 (CSA, 2022; Tadesse et al., 2022). Globally, wheat production faces significant challenges, as demand is expected to increase the wheat supply by about 50% as the world population is predicted to approach 10 billion people by 2050 (Figueroa et al., 2018, United Nations, 2022).
However, an immense imbalance exists between wheat production and supply due to increasing demand associated with significant urban population expansion. To counterbalance the deficit, we imported 1.5 million tonnes of wheat on average for 700 million dollars each year over the last five years. Nonetheless, there is substantial opportunity to enhance wheat production but it is significantly hampered by multiple biotic and abiotic factors. The unprecedented worldwide climate change has severely impacted our environment and engendered severe threats to wheat productivity which has led to the emergence of new races and epidemics of pathogens (Rajpal et al., 2023). The overwhelming influence of biotic factors (pathogens like fungi (rusts, septoria, Fusarium head blight), viruses, bacteria, and nematode)) that may contribute to average global losses of 21.5% of wheat yield (Savary et al., 2019; Tadesse et al., 2022). Amongst fungal pathogens, Fusarium head blight (FHB) also called scab, is one of the major devastating and dangerous necrotrophic diseases of wheat with different fungal species from the genus Fusarium affecting wheat production worldwide (Parry et al., 1995; Summerell, 2019; Alisaac et al., 2023; Tiffany et al., 2023). The pathogens may infect a number of cereal crops including wheat, barley, oats, rye, corn, rice, canary seed and forage grasses particularly, the most affected crops are wheat, barley and maize (Ruan et al., 2020). Durum wheat is extremely vulnerable to FHB due to the source of resistance being rare in the primary gene pool and the morphological nature of the crop compared to bread wheat, barley and oats (Jemanash et al., 2019).
On the global scale, Fusarium head blight (FHB) is considered the most dangerous and destructive fungal disease of wheat that generates the greatest economic losses, especially in humid and semi-humid wheat-growing regions (Tang et al., 2022; Okorski et al, 2022). Over the last few years, the frequency of FHB epidemics has been substantially increasing worldwide particularly, in Ethiopia high epidemics occur during the 2022 main cropping season. In Ethiopia, climate change as well as changes in farming systems allowed FHB to gradually spread throughout the regions, where it is now a great headache to the main wheat production area (Tang et al., 2022; Abdissa et al., 2022; Getachew et al., 2022; Muluken et al., 2022; Zerihun et al., 2023).
FHB resistance is quantitative, influenced by environmental factors, with significant genotype-environment interactions. Severe epidemics of the disease have occurred when virulent strains of these pathogens coincide with favorable environmental conditions and susceptible hosts with vulnerable crop growth stages (Abdissa et al., 2022; Zhang et al., 2022). Fusarium head blight (FHB) is a cosmopolitan that occurs in all continents (except Antarctica) and a monocyclic fungal disease that overwinters on wheat residues which serve as the primary inoculum for the pathogen development in the following year (Leplat et al., 2012; Reis et al., 2016; Yerkovich et al., 2020; Miedaner et al., 2023). The primary inoculum infects plants during the growing season, resulting in the primary infection. Then, the fungus spores formed on the infected tissues can cause secondary infection. The infections occur primarily during the anthesis stage and shortly afterward when warm, humid weather prevails and the infected plants cannot be treated and cured (Yingxin et al., 2022).
FHB is best known as a disease affecting flowers, with anthers as the primary infection site where fungus spores land and grow into spikelets; the concentration of overwinter fungi, and the primary infection intensity are highly correlated with temperature and vegetation vigour. FHB causes significant yield losses and wheat yield loss due to FHB results mainly; poor seed germination and discoloration, reduced seed weight and seed quality, shrivelling of kernels and kernel size, low protein content and low baking quality, reduced number of kernels per spike and contamination with mycotoxins (Dahl and Wilson, 2018; Wilson et al., 2018). Yield losses due to the FHB can reach up to 80% of the crop (Matthies and Buchenauer, 2000; Alisaac et al., 2023). None of the management strategies is completely effective by itself, and an integrated approach incorporating multiple control methods simultaneously is the only effective strategy to control FHB and reduce deoxynivalenol (DON) contamination in human food and animal feed chains (Wegulo et al., 2015; Torres et al., 2019). This review summarizes the FHB disease complex with the corresponding mycotoxin profiles, disease symptoms and life cycle, diagnostic methods, the current status of FHB epidemics, and the management strategy of the disease.
Current status, epidemics and geographical distribution of Fusarium head blight
FHB is a catastrophic and dangerous fungal disease of wheat because of its ability to cause the complete annihilation of wheat spikelets and great headache to wheat production worldwide(Okorski et al., 2022). It has emerged as one of the main hazards to global wheat production in the past three decades with an increasing trend of epidemics. In 1884, Smith from England was the first to describe the wheat disease known as Fusarium head blight (Smith, 1884). Later, it spread to other regions of the world and developed into a highly destructive disease for wheat and barley crops produced in humid and semi-humid regions, including North Central America, Canada, Asia, Eastern and Western Europe, Australia, China, Russia, Brazil, Romania, India, France, and South America (Dickson, 1942; Scott, 1986; McMullen et al., 1997; Ban et al., 2006; Muthomi et al., 2007, McMullen et al., 2007, McMullen et al., 2012; He et al., 2013). In Germany, about 70% of total arable land is potentially affected by Fusarium head blight and around 60% in Austria (Miedaner et al., 2023).
Severe FHB outbreaks have been reported every 4 to 5 years in the USA, China, the European Union (EU), Great Britain, and Brazil (Figueroa et al., 2018). In the United States (U.S.), its outbreaks resulted in losses of 288,000 metric tonnes in 1917, 2.18 million metric tonnes in 1919, 2.72 million metric tonnes in 1982, 4.78 million metric tonnes in 1993 (McMullen et al., 1997), and 1.3 million metric tonnes between 1998 and 2000 (Nganje et al., 2002). In monetary terms, the United States lost a total of $ 7.7 billion in wheat and barley production from 1993 to 2001 (Nganje et al., 2004), and from the late 1990s to the early 2000s, the United States lost a total of $ 2.7 billion in wheat and barley production owing to FHB (Nganje et al., 2004). Likewise, other important wheat-producing countries such as China, Russia, India, and France had FHB epidemics with seasonal and regional variations. In China, from 1950 to 2003, 9 severe and 17 medium epidemics occurred along the mid-lower levels of the Yangtze River, covering an area of 4 million hectares of wheat (Bai and Shaner, 1994, Parry et al., 1995, McMullen et al., 1997, Zohary et al., 2012, Zhang et al., 2013, Wang et al., 2015). Long-term wheat-maize rotation, increased implementation of reduced tillage, and highly sensitive wheat cultivars have been the main reasons for FHB's rapid expansion in China (Zhu et al., 2018; Tang et al., 2022).
In Ethiopia, FHB of wheat was described as one of the key wheat diseases in 1985 at high-altitude areas where the climate is cold and wet (Eshetu, 1985). Later, in 1989, it was become an eminent wheat disease, causing yield losses of 60% or more in experimental plots (Snijders, 1989). For many years, FHB was not regarded as a major problem in wheat production in Ethiopia. Nowadays, it has become one of the most destructive diseases of wheat during wet, warm, and high rainfall periods from anthesis to the soft dough growing stage, and epidemics are primarily initiated by initial inoculum from infected crop residue (Kebede, M., et al., 2021; Getachew et al., 2022; Zerihun et al., 2023).
Bekele (1990) identified Fusarium head blight species for the first time in Ethiopia. From stored wheat grains and blighted wheat heads, he identified F. avenaceum, F. graminearum, F. poae, F. lateritium, F. sambucinum, F. semitectum, F. sporotrichioides, F. udum and F. heterospoum (Bekele, 1993). Minhayil et al., (2021) reported that 12 Fusarium species was identified in southwestern Ethiopia during the 2017 main season based on their cultural and microscopical characteristics, namely: F. graminearum, F. culmorum, F. poae, F. avenaceum, F. ussurianum, F. semitectum, F. lateritium, F. sambucinum, F. pseudograminearum, F. heterosporum, and F. udum. Getachew et al. (2022) also reported that 9 Fusarium species, including: F. graminearum, F. culmorum, F. avenaceum, F. poae, F. ussurianum, F. semitectum, F. lateritium, F. sambucinum, and F. heterosporum, were identified from SNNP, Ethiopia during the 2019 main season.
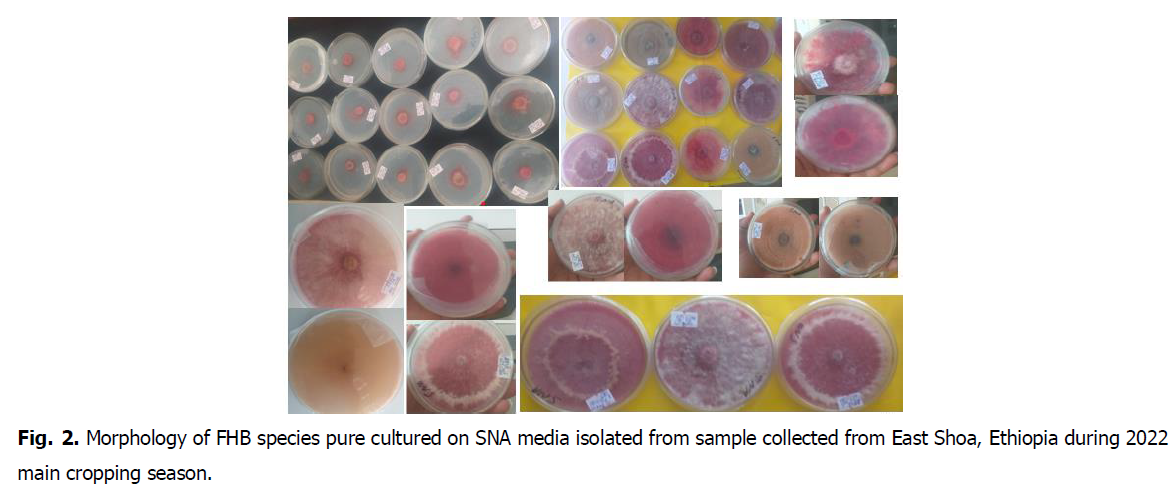
Furthermore, in the 2022 main cropping season, we had collected samples of Fusarium head blight infected wheat spikes from the East Shoa, North Shoa, and Arsi, Ethiopia. The pure cultured isolates were done in our laboratory and the isolates sent to USAD Minnesota University. A total of eleven (11) Fusarium species were identified, namely: Fusarium graminearum, F. avenaceum, F. boothii, F. equiseti, F. guttiforme, F. sp.strain, F. verticilliodes, F. arcuatisporum, F. hainanense, F. iranicum and F. pseudocircinatum. Of the identified species F. graminearium and F. equiseti were the most dominant and followed by F. boothii. Six (6) of the Fusarium species detected (54.5%) had not previously been reported in Ethiopia, while the remaining 45.5% had been previously described by other researchers (unpublished data) (Fig. 1 and Fig. 2).
Fig. 1. Fusarium Head Blight infected field at East Shoa, Ada’a district (Dire Shoki kebele) and Lume district (sherra dibendiba kebele), Ethiopia during 2022 main cropping season.
Fig. 2. Morphology of FHB species pure cultured on SNA media isolated from sample collected from East Shoa, Ethiopia during 2022 main cropping season.
Taxonomy and biology of Fusarium head blight
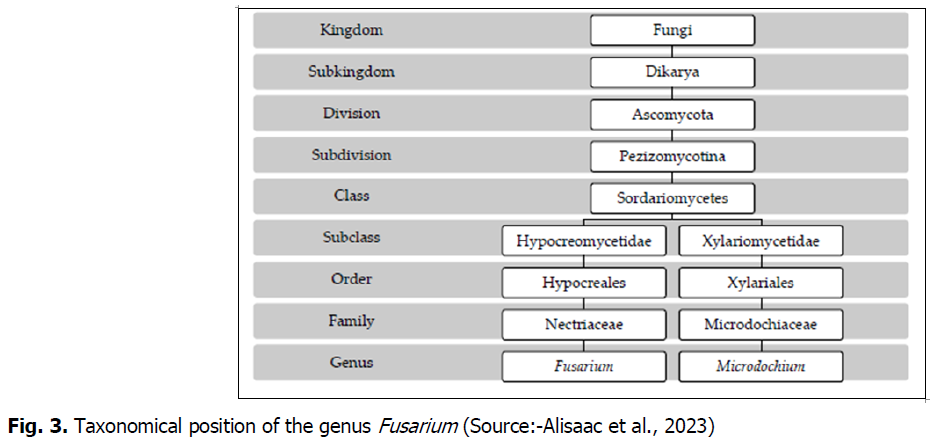
The FHB is caused by members of several complex Fusarium species; which comprises more than 19 species (Boutigny et al., 2011). Fusarium is classified in the kingdom: Fungi, Subkingdom: Dikarya, Phylum/Division: Ascomycota, Class: Ascomycetes, Family: Nectriaceae, Order: Hypocreales and Genus: Fusarium (Leslie, 1995; Alisaac et al., 2023), while Fusarium teleomorphs are mainly classified in the genus Gibberella, and for a smaller number of species, in the Hemanectria and Albonectria genera (Moretti, 2009). More recently, an extensive investigation was reported about 116 species under the genus Fusarium (Refai et al., 2015). The most prevalent species, F. graminearum (teleomorph Gibberella zeae), has recently been ranked fourth among plant fungal diseases in terms of scientific and commercial importance (Dean et al., 2012) (Fig. 3).
Fig. 3. Taxonomical position of the genus Fusarium (Source:-Alisaac et al., 2023)
Symptoms and life cycle of Fusarium head blight
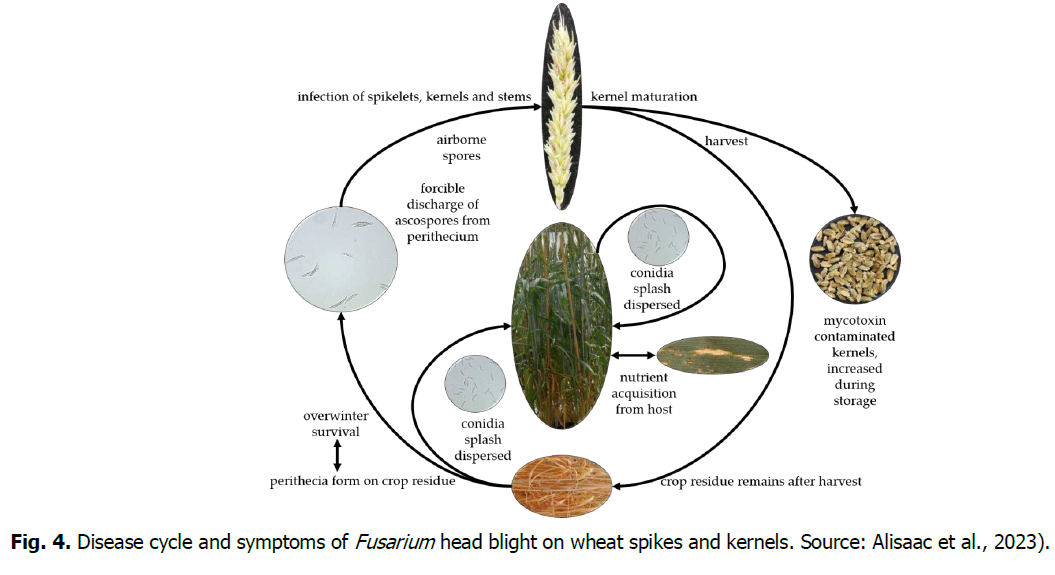
FHB symptoms on wheat spikes are most noticeable during flowering (anthesis; Feekes 10.51; Zadoks 61). Early mature bleaching of infected spikelets and the production of orange sporodochia at the base of glumes are characteristics of FHB. As the fungus invades tissues within the head, entire heads may be killed promptly (Bilikova and Hudec, 2013). As FHB progresses, brown to grey areas may appear along the stem behind the heads (peduncle) (Tom et al., 2021). Warm, humid, and wet conditions promote the development and spread of FHB in small grain crops. When these weather conditions exist prior to and during the anthesis stage, the probability of infection by the FHB pathogen is enormous. It infects wheat heads during flowering, with a short symptomless biotrophic phase of infection preceding a necrotrophic phase of disease. When the weather is dry and the humidity is low, the danger of infection during anthesis is reduced. (Tom et al., 2021). The fungus will cause kernels to shrivel and eventually be under-developed, or the FHB-fungus can colonize the outside of the kernel with no obvious symptoms yet result in the production of DON (Tom et al., 2021). In subsequent seasons, the main sources of FHB infection have been saprotrophic mycelia in crop residues from small grain crops and corns, as well as chlamydospores and conidia disseminated by wind, rain, and insects during the flowering stage (Leslie et al., 2021). During the flowering phases of wheat, infections by the FHB fungus develop during extended periods of warm, wet, and humid weather (48 to 72 hours). FHB can be caused by two types of spores. Fungal spores germinate on the surface of spikelets, and the mycelium penetrates spikelets passively through the stomata or actively through the cell walls. Sexual spores (ascospores) from residue are the primary source of inoculum and are wind and rain-dispersed to open flowers in small grain crops. Asexual spores (conidia) from host residue do not travel long distances and are dispersed by rain splash. Regardless of the source, spores land on the flowers and germinate; subsequently, the fungus grows into the developing kernel. Depending on the level of resistance in the variety of the small grain crop, the fungus may continue to colonize other kernels in the head. If the environment remains conducive, the fungus continues to grow and sporulate resulting in pale pink or salmon-colored masses (sporodochia) (Tom et al., 2021) (Fig. 4).
Fig. 4. Disease cycle and symptoms of Fusarium head blight on wheat spikes and kernels. Source: Alisaac et al., 2023).
Fusarium head blight diagnosis on wheat
Accurate disease diagnosis and precise identification of any pathogens involved is an essential prerequisite for understanding plant diseases and controlling them effectively. Traditional methods of identifying plant pathogens can be slow and inconclusive, and this has prompted the search for alternative diagnostic techniques (Ward et al., 2004). Erroneous disease detection increases the use costs of pesticides and pollutes farmland, emphasizing the need for FHB detection in wheat fields (Zhang et al., 2022). There are various approaches for identifying the fungal pathogens involved in FHB on wheat. The conventional approach involves re-isolating pathogens on selective media and identifying the fungus based on the morphological characteristics of the spores or colony. The immunological technique uses particular antibodies against a fungus-produced protein or protein complex. The most specific method, however, is the molecular method, which employs specific primers that target a specific region in the fungus's DNA. Traditional FHB detection mainly relies on professionals to scout the development of wheat infection through visual interpretation, or scholars use chemical methods, such as gas chromatography (GC) (Simsek et al., 2012), high-performance liquid chromatography (HPLC) (Simsek et al., 2012), enzyme-linked immunosorbent assay (ELISA) (Maragos et al., 2006) and polymerase chain reaction (PCR) (Amar et al., 2012) to detect FHB and DON production (Zhang et al., 2022). Fusarium species can be identified based on the visual and microscopic characteristics of the colony and spores after re-isolating the fungus on a selective media Malchet-Green Agar (MGA), Czapek Dox iprodione dichloran agar (CZID), dichloran chloramphenicol peptone agar (DCPA), Spezieller Nahrstoffarmer Agar (SNA), modified Czapek Dox agar (MCz), Nash and Snyder medium (NS) are selective media while Potato Dextrose Agar (PDA) is a general media used to isolate Fusarium species. MGA 2.5, on the other hand, was suggested as a selective medium for Fusarium re-isolation from naturally infected kernels (Bragulat et al., 2004). Furthermore, based on their pigmentation on CZID, Fusarium species could be identified (Thrane, 1996). Recently, various mediums containing the bacterial toxin "toxoflavin" produced by Burkholderia glumae demonstrated selectivity to Fusarium species (Jung et al., 2013). However, this procedure is tedious and time-consuming, and it requires experts in fungal taxonomy to diagnose the disease at the species level. Enzyme-linked immunosorbent assay (ELISA) is used as a diagnostic method for Fusarium using poly-or monoclonal antibodies. These antibodies are obtained after immunization of animals or cell lines by exoantigens secreted by Fusarium. However, the main drawback of this method is that it is genus-specific (Brunner et al., 2012). The polymerase chain reaction (PCR) allows the detection of plant diseases before the symptoms become visible. Moreover, it differentiates between fungal species scales even when they have morphological similarities. Different primers were developed to detect Fusarium species involved in FHB (KuzdraliÅ?ski et al., 2017).
Integrated management of Fusarium head blight
The management options so far recommended for the control of disease include cultural practices, cultivar resistance, application of fungicides and integrated management. The use of resistant varieties against complex Fusarium species still remains the most effective, durable, environmentally safe and economically feasible strategy for managing the disease and associated mycotoxin contamination (Wegulo et al., 2015; Abdissa et al., 2022; Getachew et al., 2022). The host response to infection and disease development varies widely. Genetic resistance to FHB is generally expressed as a quantitative trait, presumably due to many minor genes and few major ones conferring the resistance and as such, there is wide variation in phenotypic reaction and environmental response. FHB is extremely difficult to predict and control, so a multi-pronged approach is most effective. The effective management of FHB is challenging due to several factors. Firstly, maize intensification and reduced tillage increased the frequency of FHB epidemics during the last decades. This is because maize is the main host of Fusarium species, which serves as a source of the inoculum, and reduced tillage helps to keep this source available during wheat vegetation. In addition, wheat comes very often after maize in the crop rotation, which increases the disease incidence during the availability of the inoculum. Secondly, the visible FHB symptoms appear on wheat spikes at a later stage of pathogenicity, and during this stage, it is too late for fungicide application because the kernels have been contaminated with Fusarium mycotoxins. In addition, FHB control using fungicides involves different disadvantages mainly costs, bio-and eco-hazards, relatively short lifetime due to fungicide resistance, and low availability for smallholder farmers. Furthermore, environmental and health protection measures necessitate ongoing regulatory adjustments in terms of fungicide availability and applicability (Nelson, 2020). This demonstrates the importance of an integrated disease management strategy that includes cultural practices, resistant cultivars, biological control and chemical seed treatments.
Cultural practices
Crop rotation
FHB can survive in crop residues, therefore, properly designed crop rotation. To reduce the buildup of infested crop residues, rotating away from cereals particularly maize crops to non-host crops, including pulses and forage legumes, should be considered for at least 2 years. This will allow enough time for infested residue to decompose before the next cereal crop is planted (Islam et al., 2022).
Use clean seed
High-quality clean seed is an important element in preventing the occurrence of pathogenic fungi, such as Fusarium spp. and their metabolites in plant cultivation. Seeds should be healthy, without signs of damage that could facilitate pathogen penetration, and they should have adequate viability. Where possible, producers must avoid planting the seed that is infected with Fusarium. Seed of susceptible crop species must be tested by a seed testing laboratory and only seed with non-detectable levels of Fusarium species is to be used for seeding purposes. Although infected seed can cause seedling blight, it typically does not directly give rise to head blight symptoms in one growing season. To prevent or reduce damping off and seedling blights, scabby grain should be thoroughly cleaned and treated with a systemic fungicide before being used as seed for next season’s crop. The fungus will move from the infected seed to the root, crown and stem base tissues of the plant that develops from the infested seed, therefore, creating potential sources of infested residue that can impact subsequent crops. The buildup of the pathogen would also be favoured by growing successive host crops continuously or in short rotations, and disease-conducive weather (Moya-Elzondo and Jacobsen, 2016).
Increase seeding rate
Increasing seeding rate causes less tillering leading to a more uniform and shorter overall flowering period which minimizes the length of time during which heads are susceptible to FHB infection. Less tillering means less variation in the crop growth stage, which may improve overall fungicide performance. Less tillering and a shorter flowering period also reduce the time that irrigation should be avoided (during the flowering period) when the pathogen infects wheat and barley crops (Schaafsma and Tamburic-Ilincic, 2005).
Modification of planting dates
Staggering planting dates to avoid having all cereal fields flowering at the same time is very important and modification of planting date is also an important element in preventing the occurrence of Fusarium spp. and their metabolites. The planting date determines the flowering date & environmental conditions at flowering are critical for the occurrence of FHB (Gorczyca et al., 2018). The risk of plant infection by Fusarium species, and thus contamination with mycotoxins is always greatest when the flowering period of a given plant is close to the date of fungus spore release. Appropriate planting date range and changing the heading time of the plant (escaping) are one of the ways to prevent the FHB epidemic (Hossein, 2017). Changes in the phenology of wheat cultivars under some climate change scenarios could significantly increase FHB and DON accumulation.
Host plant resistance
Developing resistant cultivars is the most effective and economical to minimize losses caused by the FHB. The host response to infection and its development varies widely due to variations in phenotypic reaction and environmental response. Resistance to FHB is governed by multiple Quantitative Trait Loci (QTL) and is highly influenced by changing environments. Genetic resistance to FHB is expressed as a quantitative trait, due to many minor genes and few major ones conferring the resistance (Wegulo et al., 2015). More than 400 resistant Quantitative Trait Loci (QTL) have been identified so far mainly located on chromosomes 5A, 3B, 6B, 6D, and 7D (Jia et al., 2018; Ma et al., 2020). Evaluating FHB resistance is often not possible by natural infection as disease intensity varies over time due to changes in the environment (Mesterhazy et al., 2003). Obtaining consistent differentiation of FHB resistance levels relies on the use of inoculation methods (Parry et al., 1995). Moreover, the deployment of resistant genotypes is ideal in terms of effectiveness, eco-friendliness, and sustainability of production (Getachew et al., 2020).
Components of wheat resistance to FHB include passive resistance represented by morphological and phenological features and active resistance represented by physiological features (Mesterházy,1995). Morphological and phenological features that are involved in passive resistance are plant height, wheat awns, narrow and short floral openings, and the time of retained anthers. Plant height: tallness helps wheat spikes to stand away from splashed rain droplets that carry the inoculum from the soil surface and crop residues. Wheat awns: awns trap the inoculum and increase natural infection while their absence reduces it (Mesterházy, 1995). A narrow and short floral opening reduces the floret’s exposure to the inoculum and increases resistance while retained anthers and pollen might trap the inoculum and catalyze spore germination and fungal penetration (Steiner et al., 2017). Resistance can be classified into the following types: resistance to initial penetration or infection [Type I resistance] Mesterhazy et al., (1995), resistance to fungal spread within the spike from the infected spikelet [Type II resistance] (Schroeder et al., 1963), resistance to mycotoxin accumulation [Type III resistance] (Miller et al., 1983), resistance to kernel infection [Type IV resistance] and tolerance to yield loss [Type V resistance] (Mesterhazy et al., 1995). Type IV and type V can be merged because both reflect grain disease resistance (Gong et al., 2020).
Chemical control
Effective chemical control of FHB should be combined with other management practices and the triazoles class of chemical fungicides in the Demethylation Inhibitor (DMI) fungicide group that inhibits sterol biosynthesis, are the most effective fungicides for suppressing FHB symptoms and reducing mycotoxin levels (Wegulo et al., 2015). According to Paul et al., (2018), the most effective treatment for reducing FHB index and DON was to apply DMI fungicides to wheat anthers at the Feekes 10.5.1 growth stage. Previous research has reported on the successful reduction of FHB severity and DON concentrations, and thus reduced yield and quality losses, from the timely application of triazole-based fungicides (Palazzini et al., 2017). Cromey et al. (2001) found that applying tebuconazole to FHB-infected wheat plants reduced FHB incidence by up to 90% and increased yield by 14%. Meta-analyses of fungicide trials conducted in the United States also revealed that metconazole, prothioconazole+tebuconazole, and prothioconazole were the three most effective fungicide treatments in terms of yield and test weight increase (Paul et al., 2018). Demethylation inhibitor (DMI) fungicides, namely tebuconazole, metconazole, prothioconazole, and prothioconazole+tebuconazole are effective triazole fungicides for reducing FHB infections and deoxynivalenol (DON) levels (Mesterházy et al., 2011; Freije and Wiese, 2015). The timing of fungicide application is also critical for FHB control. Hence, applying fungicides at an early stage of pathogenicity should be considered in the management strategy (Alisaac et al., 2023). Integrated disease management strategies are regarded as the best way to control FHB due to the greater reduction in FHB severity and DON concentrations that could be achieved (Schoeman et al., 2017).
Seed treatment
Seed treatment is an important component of integrated disease management for producing small-grain cereals. It is the most effective way to protect wheat against FHB (Moya-Elzondo and Jacobsen, 2016; Getachew et al., 2022). Though unable to prevent infection afterwards in the growing period, chemical seed treatment help to prevent seedling blight caused by Fusarium species, they involve in escaping the seedlings from becoming blight and dead during the early stage of the crop. Fungicides namely, Carbendazim 75% WP, Imidalm T 450 WS, Tebuconazole 2 DS, Difenoconazole 25% EC, Propiconazole 25% EC, Thiram 50% WP, Carboxin 37.5%+Thiram 25%, Torpedo (Thiamethoxam+Metalaxyl-M), Pyraxonil 30 FS (Clothianidin 25%+fludioxonil 2.5%+pyraclostrobin 2.5% FS) and Apron Star WS (Thiamethoxam 200g/kg+Metalaxyl-M 200g/kg+Difenoconazole 20g/kg are registered and currently used as a seed treatment (Ram et al., 2021; Getachew et al., 2022). Fungicide seed treatments are designed to mitigate external or internal microorganisms from seeds or soil, resulting in healthy seedlings and plants (Khanzada et al., 2002; Beres et al., 2016). Thus, seed can be treated to promote good stand establishment, minimize yield loss due to suboptimal seed quality, and limit the spread of pathogens, although fungicide seed treatment does not completely eliminate the risk of disease transmission, damage from the latter pathogens can be more severe than if the seed had not been treated (Richard et al., 2002; Beres et al., 2016; Turkington et al., 2016).
Mycotoxins
Mycotoxins are secondary metabolites of microscopic fungi that commonly contaminate cereal grains (wheat, barley, oat, rye, maize and rice) and their products (Elzbieta and Barbara, 2020). Globally, about 1 billion metric tons of food and food products are lost due to mycotoxin contamination every year (Schmale and Munkvold 2009). Annually, 25-50% of crops harvested worldwide are contaminated with mycotoxin (Ricciardi et al., 2013). Eskola et al., (2020) reported that globally mycotoxins contaminate up to 80% of agricultural products (Eskola et al., 2020). It causes a wide range of harmful health effects and poses severe health risks to humans and livestock, among others they are mutagenic, teratogenic and estrogenic. The adverse health effects of mycotoxins range from acute poisoning to long-term effects such as immune deficiency and cancer on human beings. Fusarium species cause FHB are common to produce a range of different toxins, such as deoxynivalenol (DON), nivalenol (NIV), T-2 and HT-2 toxins, as well as zearalenone (ZEN) and fumonisins. Different Fusarium toxins are associated with certain types of cereal crops (Mawcha et al., 2022). For example, DON, NIV and ZEN are often associated with wheat, T-2 and HT-2 toxins with oats, and Fumonisins with maize. The U.S. Food and Drug Administration has established a 2-ppm threshold for DON in wheat grain, a 1-ppm limit for finished wheat products that humans may consume, and 5 to 10-ppm for grains and grain by-products destined for livestock feed. As a result, harvesting grain with high levels of DON may lead to price discounts or rejections at the elevator (FDA, 2018).
Excessive production of mycotoxins is extremely vulnerable during the epidemic outbreak of FHB. Mycotoxin levels should be monitored routinely and continuously, as the annual levels may vary depending on environmental moisture, climate, temperature changes, plant disease status, and insect pest numbers. Effective management of food safety risks is required, especially including the use of rapid and sensitive immunological techniques (Ji et al., 2019). Decreasing mycotoxin contamination has become one of the targets for FHB resistance breeding (Xian et al., 2022). The occurrence of FHB and associated mycotoxins vary among seasons hence the need for continuous monitoring and surveillance of the disease and associated toxins
Types and toxicities of Fusarium head blight mycotoxins
Fusarium species produce the three most important classes of mycotoxins namely: trichothecenes, zearalenone (ZEN), and Deoxynivalenol (DON).
Deoxynivalenol (DON)
Deoxynivalenol (DON) known as vomitoxin is the first and most common contaminant of cereal grains worldwide. It is produced by the fungus to facilitate the spread of the fungus through the rachis and to adjacent spikelets and grains (Valenti et al., 2023). The ingestion of DON in mammals can result in acute toxic effects such as nausea, gastroenteritis, vomiting, diarrhea, and increased salivation. In addition, chronic toxic effects such as immunotoxicity, altered nutritional effects, weight loss, and anorexia have been frequently observed. In dairy cattle, it has been linked to reduced milk. Deoxynivalenol is unlikely to appear as residues in the tissues or fluids of animals exposed to toxic levels, but baking and malting using DON-contaminated wheat and barley can have adverse effects. DON is a potent protein synthesis and cell division inhibitor and causes a significant mitosis reduction, especially in wheat crops. It strongly inhibits coleoptile and shoot elongation and also negatively affects root growth in wheat (Wang et al., 2020; Ederli et al., 2021).
Trichothecenes
Trichothecenes are the most dominant and virulent group of Fusarium mycotoxins accompanying FHB infection on wheat worldwide (Foroud et al., 2019). It is a global concern usually consumed by livestock and humans (Eriksen and Petterson, 2004). This group is split, based on its chemical structure, into four subgroups A, B, C, and D (Chen et al., 2019). However, trichothecenes produced by Fusarium spp. are A and B. The main difference between these two groups is the presence of ketone (=O) at C8 of trichothecenes backbone in trichothecenes B while it is absent in trichothecenes A (Foroud et al., 2019). In general, trichothecenes A are more toxic in animals compared with trichothecenes B; however, in crops, trichothecenes B are more toxic.
Trichothecenes A includes T-2 toxin, HT-2 toxin, diacetoxyscirpenol (DAS), monoacetoxyscirpenol (MAS), neosolaniol (NEO), NX-2 and NX-3. This group is mainly produced by F. acuminatum, F. equiseti, F. graminearum, F. poae, F. sambucinum, and F. sporotrichioides. Trichothecenes B includes nivalenol (NIV), 4-acetyl-nivalenol (4-ANIV), deoxynivalenol (DON), 3-acetyl-deoxynivalenol (3-ADON) and 15-acetyl-deoxynivalenol (15-ADON). Fusarium species that produce trichothecenes B are F. acuminatum, F. crookwellense, F. culmorum, F. equiseti, F. graminearum, F. poae, F. sambucinum, F. semitectum, and F. sporotrichioides. However, DON is more poisonous in crops while NIV is more poisonous in animals (Ferrigo et al., 2016). Trichothecenes are potent inhibitors of eukaryotic protein synthesis, interfering with initiation, elongation, and termination stages. Some of the diseases associated with these toxins in humans and animals include feed refusal, nausea, vomiting, abortions, weight loss, inflammation of the skin, hemorrhaging of internal organs, blood disorders, immunosuppression, and disturbance of the nervous system (Desjardins, 2004; Ekwomadu et al., 2021).
Zearalenone (ZEN)
Zearalenone, often known as F-2, is a commonly contaminate maize and also one of the most prevalent Fusarium mycotoxins on wheat around the world (Ekwomadu et al., 2021). Zearalenone derivatives, mainly, zearalanone, α-and β-zearalenol, and α-and β-zearalanol could be naturally produced by Fusarium spp. (Ferrigo et al., 2016). The main difference is the presence of ketone (=O) at C12 in zearalenone and zearalanone while it is hydroxyl (-OH) in α-and β-derivatives. Zearalenone is of low acute toxicity either in Planta or in Animalia compared with trichothecenes (Mclean, 1995). Fusaria involved in zearalenone production are F. crookwellense, F. culmorum, F. equiseti, F. graminearum, F. semitectum, and F. sporotrichioides. In Animalia, zearalenone has an estrogenic effect by binding to estrogen receptors which affects the sexual activities of animals (Bertero et al., 2018). The consumption of contaminated grains by farm animals can lead to the manifestation of female features in males, early sexual development of young females, infertility in adults, abortion, false heat, recycling, stillbirth, the birth of malformed offspring, reabsorption of fetuses and mummies (Ekwomadu et al., 2021).
Detection of Fusarium head blight mycotoxins
Mycotoxins can be detected by various techniques, which are broadly divided into instrumental and bioanalytical methods. However, each approach has merits and drawbacks; the method:
Chromatographic methods
There are many kinds of instrumental detection methods for mycotoxins. Thin layer chromatography (TLC) is a qualitative or semi-quantitative method with the longest history in the detection of mycotoxins. High-performance liquid chromatography (HPLC) can couple with different detectors. These detectors include ultraviolet (UV) detection, diode array detection, fluorescence detection or mass spectrometric detection. Gas chromatography can couple with electron capture detection, flame ionization detection (FID), or mass spectrometry (MS) detection (Lippolis et al., 2008). These methods afford high accuracy and precision and are used for quantitative and qualitative analyses. However, they are expensive, require skilled personnel and longer periods for sophisticated sample preparation (Elliott, 2011). Thus, instrumental methods are not suitable for normal laboratories or field environments. Chromatographic techniques involving UV and FID are principally employed in confirmatory contexts, thus facilitating compliance with regulations. Occasionally, such techniques serve as reference methods for validating immunochemical tests.
Immunochemical methods
Immunoassays based on antibody-antigen reactions are very useful for routine analyses, as these techniques are simple and have been used for rapid mycotoxin detection (Zherdev, 2014). Recently, several immunological techniques have been developed, including enzyme-linked immunosorbent assays, time-resolved immunochromatographic assays, enzyme-linked aptamer assays, chemiluminescence immunoassays, fluorescence immunoassays, fluorescence resonance energy transfer immunoassays, and metal-enhanced fluorescence assays (Chauhan et al. 2016). Aptamer is an important parameter in these detection techniques. It can bind a variety of peptides, proteins, amino acids, and organic or inorganic molecules, all of which have high affinity and specificity. Liu et al., (2014) constructed an ultrasensitive immunosensor based on mesoporous carbon and trimetallic nanorattles with special Au cores. The lower detection limit of ZEN was 1.7 pg/mL, and the assay was found to exhibit good stability and reproducibility.
Because of the strong selectivity of molecular recognition mechanisms, it is difficult to simultaneously assay different compounds or discover new toxins. In comparison to chromatographic methods, immunochemical methods afford greater selectivity in terms of monitoring mycotoxin levels which is very important to ensure food safety in developing countries. In addition, due to global changes in climate and the environment, the level of contamination by fungi and their mycotoxins will increase in the future. Risk management requires the routine application of efficient control programs such as optimally employing immunoassays (Ji et al., 2019).
Conclusion
FHB is an extremely harmful and devastating fungal disease. Weather environments, particularly rainy days with mild temperatures during anthesis and an abundance of primary inoculum, are substantially connected with the occurrence of FHB outbreaks. The Fusarium head blight epidemic in wheat throughout the world is a serious danger that must be controlled before it causes enormous damage and human suffering. It has been demonstrated that combining multiple control methods is an effective approach in the integrated disease management of FHB. Management strategies must be considered both before and after wheat planting. Applying appropriate cultural practices, demethylation inhibitor (DMI) fungicides group and planting resistant varieties plays an important role in minimizing disease incidence and severity. Predicting and monitoring the disease, on the other hand, will aid in the decision to use biological and chemical control during the growing season.
References
Abdissa, T., Bekele, B. (2020). Assessment of wheat Fusarium head blight and associated Fusarium species in West Shewa, Ethiopia. International Journal of Agriculture and Biosciences, 9:299-304.
Abdissa, T., Bekele, B., Selvaraj, T. (2022). Evaluation of Response of Wheat (Triticum spp) genotypes against Wheat Fusarium Head Blight in Ethiopia. Advances in Crop Science and Technology, 10:505.
Alisaac, E., Mahlein, A.K. (2023). Fusarium head blight on wheat: Biology, modern detection and diagnosis and integrated disease management. Toxins, 15:192.
Google Scholar, Crossref, Indexed at
Amar, A.B., Oueslati, S., Ghorbel, A., Mliki, A. (2012). Prediction and early detection of mycotoxigenic Fusarium culmorum in wheat by direct PCR-based procedure. Food Control, 23:506-510.
Google Scholar, Crossref, Indexed at
Beres, B.L., Turkington, T.K., Kutcher, H.R., Irvine, B., Johnson, E.N., O'Donovan, J.T., Spaner, D.M. (2016). Winter wheat cropping system response to seed treatments, seed size, and sowing density. Agronomy Journal, 108:1101-1111.
Google Scholar, Crossref, Indexed at
Bertero, A., Moretti, A., Spicer, L.J., Caloni, F. (2018). Fusarium molds and mycotoxins: Potential species-specific effects. Toxins, 10:244.
Google Scholar, Crossref, Indexed at
Boutigny, A.L., Beukes, I., Viljoen, A. (2011). Head blight of barley in South Africa is caused by Fusarium graminearum with a 15-adon chemotype. Journal of Plant Pathology, pp:321-329.
Google Scholar, Crossref, Indexed at
Bragulat, M.R., Martinez, E., Castella, G., Cabanes, F.J. (2004). Selective efficacy of culture media recommended for isolation and enumeration of Fusarium spp. Journal of Food Protection, 67:207-211.
Google Scholar, Crossref, Indexed at
Brunner, K., Farnleitner, A., Mach, R.L. (2012). Novel methods for the quantification of pathogenic fungi in crop plants: quantitative PCR and ELISA accurately determine Fusarium biomass. Plant Pathology; Cumagun, CJR, Ed.; IntechOpen Limited: London, UK, pp:203-218.
Chauhan, R., Singh, J., Sachdev, T., Basu, T., Malhotra, B.D. (2016). Recent advances in mycotoxins detection. Biosensors and Bioelectronics, 81:532-545.
Google Scholar, Crossref, Indexed at
Chen, Y., Kistler, H.C., Ma, Z. (2019). Fusarium graminearum trichothecene mycotoxins: biosynthesis, regulation, and management. Annual Review of Phytopathology, 57:15-39.
Google Scholar, Crossref, Indexed at
Cromey, M.G., Lauren, D.R., Parkes, R.A., Sinclair, K.I., Shorter, S.C., Wallace, A.R. (2001). Control of Fusarium head blight of wheat with fungicides. Australasian Plant Pathology, 30:301-308.
Google Scholar, Crossref, Indexed at
Desjardins, A.E. (2006). Fusarium mycotoxins: chemistry, genetics, and biology. American Phytopathological Society.
Ederli, L., Beccari, G., Tini, F., Bergamini, I., Bellezza, I., Romani, R., Covarelli, L. (2021). Enniatin B and deoxynivalenol activity on bread wheat and on Fusarium species development. Toxins, 13:728.
Google Scholar, Crossref, Indexed at
Meneely, J.P., Ricci, F., van Egmond, H.P., Elliott, C.T. (2011). Current methods of analysis for the determination of trichothecene mycotoxins in food. TrAC Trends in Analytical Chemistry, 30:192-203.
Google Scholar, Crossref, Indexed at
Author Info
G.H. Regasa*Citation: Regasa, G.H. (2023). Harnessing and sounding the alarm on Fusarium head blight of wheat: current status, biology, detection and diagnosis method, mycotoxins, and integrated management options. Ukrainian Journal of Ecology. 13: 1-11.
Received: 01-Jun-2023, Manuscript No. UJE-23-110344; , Pre QC No. P-110344; Editor assigned: 03-Jun-2023, Pre QC No. P-110344; Reviewed: 15-Jun-2023, QC No. Q-110344; Revised: 22-Jun-2023, Manuscript No. R-110344; Published: 29-Jun-2023, DOI: 10.15421/2023_458
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.