Research - (2021) Volume 0, Issue 0
Investigation of grain yield and drought resistance in selected wheat lines based on molecular markers
D. Khan1, I. Muhammad1, M. Shuaib2*, F. Hussain3, M. Romman4, N. Azam5, S. Abidullah6, A. Zeb6, A. Rauf6 and S. Bahadur7Abstract
The current work aims to performed and know the fifty-one wheat genotypes and evaluate for drought stress. The analysis of variance (ANOVA) was performed, which shows that all the parameters are significant. Variations in quantitative traits include Peduncle length, Spike length, Plant height, Days to 50% heading, Biological yield, Flag leaf area, Yield per plant, 1000 grains weight, Number of spikelets per spike Harvest Index were recorded. The highest peduncle length, spike length, plant height, days to 50% heading, biological yield, flag leaf area, yield per plant, spikelets per spike, 1000 grain weight, and HI were observed in 10831 (46.33), 10843 (14), 10821 (116.33), 10845 (157), 10826 (18.4), 10874 (58.46), 10854 (2.944gm), 10841(26), 11881 (52) and 10738 (24.571) respectively. Seven molecular markers were used for screening drought-tolerant genotypes. The genotypes 11861, 11860, 10850, 10815, 10819, 11866, and 10825 have amplified a maximum number of drought resistance genes as 7, 7, 7, 6, 6, 6, and 6, respectively.
Keywords
ANOVA, drought, quantitative traits, wheat.
Introduction
Wheat (Triticum aestivum L.) is the most important cereal crop for most of the world’s populations. It is the essential staple food of about two billion people (36% of the world population). Worldwide, wheat provides nearly 55% of the carbohydrates and 20% of the food calories consumed globally. Wheat-breeding programs worldwide are operational to improve grain yield with better quality, agronomic performance, and disease resistance (Zhang et al. 2011; Naeem et al., 2020). The problems of climate change affecting several crops, including wheat (increase in temperature and drought), are being addressed globally (Plaut et al. 2004, Passioura 2007, Reynolds et al. 2001, Najafian 2003 and Rajki 1982). It has been reported that about 20% of irrigated land has been affected by drought and soil salinization, and crop yield has been reduced by 20-30% throughout the world. In general, breeding for tolerance to drought involves combining good yield potential in the absence of stress and selecting traits that provide drought stress tolerance (Blum 1988). The present study tested 51 wheat varieties and detected drought-resistant varieties based on molecular markers and high grain yielding varieties based on morphological parameters.
Materials and Methods
Plant collection
The present research work was carried out under field conditions of Mansehra during 2013-2014. Fifty-one genotypes comprising germplasm and common wheat varieties (Triticum aestivum L.) collected from PGRI, and NARC Islamabad were evaluated at the morphological and molecular levels.
Primers design
Using NCBI to design the seven different primers with their base pairs, temperature, times for the 51 different wheat varieties shown in Table 1.
| S No | Primer Name | Primer Sequence | Fragment Size | Tm |
|---|---|---|---|---|
| 01 | WMC-97 | Forward | 184 bp | 51°C |
| GTCCATATATGCAAGGAGTC | ||||
| Reverse | ||||
| GTACTCTATCGCAAAACACA | ||||
| 02 | WMC-105 | Forward | 192 bp | 59°C |
| AATGTCATGCGTGTAGTAGCCA | ||||
| Reverse | ||||
| AAGCGCACTTAACAGAAGAGGG | ||||
| 03 | WMC-147 | Forward | 152 bp | 61°C |
| AGAACGAAAGAAGCGCGCTGAG | ||||
| Reverse | ||||
| ATGTGTTTCTTATCCTGCGGGC | ||||
| 04 | WMC-154 | Forward | 147 bp | 61°C |
| ATGCTCGTCAGTGTCATGTTTG | ||||
| Reverse | ||||
| AAACGGAACCTACCTCACTCTT | ||||
| 05 | WMC-169 | Forward | 167 bp | 61°C |
| TACCCGAATCTGGAAAATCAAT | ||||
| Reverse | ||||
| TGGAAGCTTGCTAACTTTGGAG | ||||
| 06 | WMC-182 | Forward | 159 bp | 56°C |
| GTATCTCACGAGCATAACACAA | ||||
| Reverse | ||||
| GAAAGTGTATGGATCATTAGGC | ||||
| 07 | WMC-219 | Forward | 180 bp | 61°C |
| TGCTAGTTTGTCATCCGGGCGA | ||||
| Reverse | ||||
| CAATCCCGTTCTACAAGTTCCA |
Table 1. Details of the primers used in the present study.
Research design, treatment, and DNA extraction Morphological studies were undertaken in the Hazara University (garden campus) research field. Fifty-one wheat germplasm was planted in Randomly Complete Block Design (RCBD) with three replications (Table 1). DNA was isolated from selected plant materials using (Weining and Langridge 1992) protocol. To check the quality and quantity, the extracted genomic DNA was electrophoresed on 1% agarose gel. After that, the gel was observed under UV light using a Uvtech system (model BTS-20.M).
PCR
Polymerase Chain Reaction (PCR) was carried out using the protocols of (Stepien et al. 2003 and Mago et al. 2002) with a little modification. The PCR was conducted for seven primers viz., wmc97, wmc105, wmc147, wmc154, wmc169, wmc182 and wmc219. The Thermo cyclic conditions were kept as initial denaturation at 94ºC for 4 min followed by 37 cycles of 94ºC for 1 min, 51 to 61ºC for 1 min, 72ºC for 2 min a final extension of 72ºC for 10 min (Figure. 1).
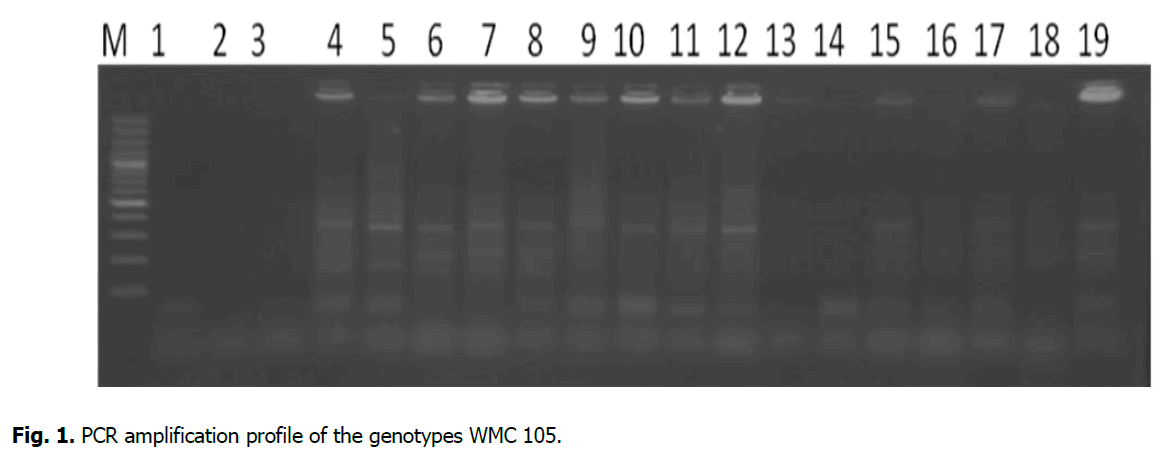
Figure 1: PCR amplification profile of the genotypes WMC 105.
Data analysis
One-way ANOVA was used to analyze the data. Fifty-one wheat germplasm was planted in Randomly Complete Block Design (RCBD) with three replications shown in (Table 1) in detail.
Results and Discussion
Fifty-one wheat varieties were studied for different morphological characters. Seven droughts specific SSR markers were also run to identify drought resistance genes.
The highest peduncle length was counted for 10831 (46.33 cm) and 10814 (45.66 cm) among morphological parameters. The highest spike length was recorded in 10843 (14 cm) and 10841 (13 cm). The highest flag leaf area was counted for 10874 (58.46 cm) and 10848 (55.06 cm). The maximum number of days to 50% heading was counted in 10845 and 10842, 157 days each. The highest plant height was counted for 10821 (116.33 cm) and 10819 (155.66 cm). The highest 1000 grain weight was counted in 11881 (52 gm) and 10874 (48 gm). Biological yield counted in 10826 and 11881, 18.4 gm each was found highest among other varieties. Among the studied varieties, 10854 (2.94) and 10814 (2.82) have the highest yield per plant. The highest number of spikelets per spike was counted in 10841 (26) and 11873 (25), while the highest Harvest Index was counted for 10738 (24.57) and 10813 (19.86). Among the fifty-one germplasm, the superior genotypes were observed based on repetition in morphological traits are 10845 (7times), 10842 and 10843 (5times), 10841 (4times), while the remaining verities are repeated at least one time.
The analysis of variance showed that all the parameters are highly significant, as shown in Table 2.
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Sum of Squares | Df | Mean Square | F | Sig | ||
| Replication | Between Groups | 0.000 | 50 | 0.000 | 0.000 | 1.000 |
| Peduncle Length | Between Groups | 8929.517 | 50 | 178.590 | 178.590 | .000 |
| Spike Length | Between Groups | 331.888 | 50 | 6.638 | 6.638 | .000 |
| Total Plant Height | Between Groups | 57658.261 | 50 | 1153.165 | 1141.860 | .000 |
| Fifty percent Heading | Between Groups | 4206.471 | 50 | 84.129 | 84.129 | .000 |
| Biological Yeild | Between Groups | 1294.289 | 50 | 25.886 | 25.886 | .000 |
| Flag Leaf Area | Between Groups | 6984.562 | 50 | 139.691 | 139.691 | .000 |
| Yield per Plant | Between Groups | 15171.529 | 50 | 303.431 | 303.431 | .000 |
| Thousand Grain Weight | Between Groups | 4670.549 | 50 | 93.411 | 1.647 | .017 |
| No of Spikelets per Spike | Between Groups | 1074.118 | 50 | 21.482 | 21.482 | .000 |
| Harvest Index | Between Groups | 3807.497 | 50 | 76.150 | 76.150 | .000 |
Table 2. Analysis of Variance for yield and yield associated traits of wheat.
The morphological dendrogram was constructed for wheat (Triticum aestivum L.) germplasms. The fifty-one wheat germplasms were sorted into seven groups as A, B, C, D, E, F, and G. The germplasm of different clusters shows the dissimilarity among genotypes and the genotypes present in the same clusters shows the similarity level. Group A was further sorted into five clusters containing 21 genotypes, group B containing three genotypes; group C has consisted of 8 genotypes, group D consisted of 3 genotypes, group E consisted of only one genotype, group F containing two genotypes. In contrast, the rest of the genotypes reside in group G (Figure. 2).
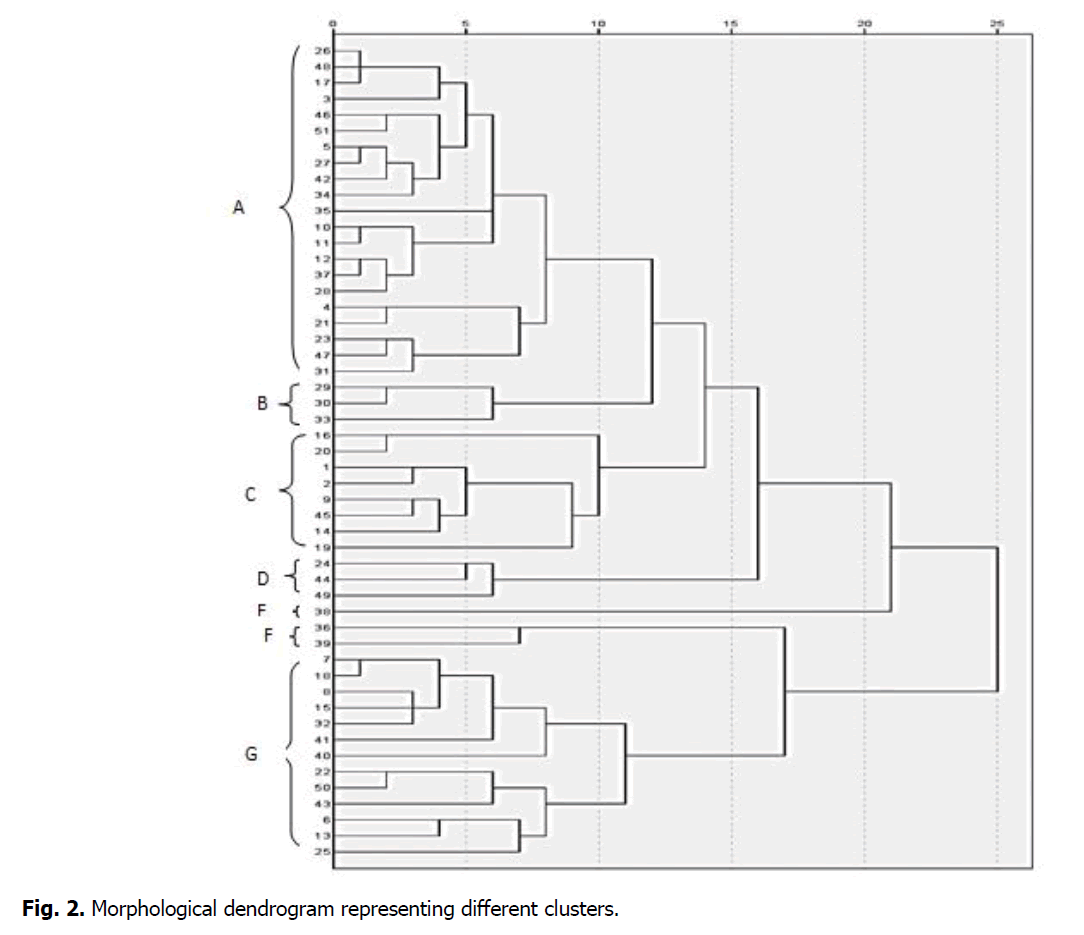
Figure 2: Morphological dendrogram representing different clusters.
The present research work following (Ashraf 2010) demonstrated that different molecular markers, currently available for tagging of different traits, are helpful for Marker-assisted breeding techniques in wheat in stress conditions and are intensively used to create stress-tolerant lines in different crops. The current study is also in close agreement with (Muhammad et al. 2016; Khan et al. 2016 and Shuaib et al., 2020), as these results suggest the usefulness of morphological studies complemented with molecular markers to enhance drought tolerance in wheat in drought conditions.
Molecular marker screening for drought tolerance
All the 51 genotypes were screened out for drought tolerance based on molecular markers. Total of seven drought markers were selected as wmc-97, wmc-105, wmc-147, wmc-154, wmc-169, wmc182 and wmc-219. The only reliable bands were included in scoring; the faint bands were not considered (Table 3). The molecular fragment size was matched with a standard molecular ladder of 100 bp (gene link Inc. USA).
| S No | Genotype | WMC219 | WMC169 | WMC97 | WMC105 | WMC147 | WMC154 | WMC182 |
|---|---|---|---|---|---|---|---|---|
| 180bpbp | 167bp | 184bp | 350BP | 152bp | 147bpS | 159bp | ||
| 1 | 11868 | + | - | - | - | - | - | - |
| 2 | 10854 | - | - | - | - | - | - | - |
| 3 | 10853 | + | - | - | - | - | - | - |
| 4 | 10850 | + | + | + | + | + | + | + |
| 5 | 10849 | + | - | + | + | + | + | - |
| 6 | 10825 | + | + | + | + | + | - | + |
| 7 | 10845 | - | - | + | + | + | + | - |
| 8 | 10847 | - | - | - | + | - | + | - |
| 9 | 11862 | + | - | + | + | + | + | - |
| 10 | 11860 | + | + | + | + | + | + | + |
| 11 | 11861 | + | + | + | + | + | + | + |
| 12 | 11866 | + | + | + | + | + | + | - |
| 13 | 11809 | - | - | - | - | - | - | - |
| 14 | 11873 | - | - | - | - | - | - | + |
| 15 | 10842 | + | - | + | + | + | + | - |
| 16 | 10841 | - | - | + | - | + | - | + |
| 17 | 10833 | - | - | + | + | + | + | - |
| 18 | 10843 | - | - | + | - | + | - | + |
| 19 | 10848 | - | + | + | + | + | + | - |
| 20 | 10832 | - | - | + | - | + | - | + |
| 21 | 10829 | + | - | + | - | + | - | + |
| 22 | 10852 | + | + | + | + | - | - | + |
| 23 | 10834 | - | + | - | + | + | - | + |
| 24 | 10831 | + | + | + | - | + | - | - |
| 25 | 10835 | - | - | - | - | - | - | - |
| 26 | 10830 | - | - | - | - | - | - | - |
| 27 | 11865 | - | - | - | - | - | - | - |
| 28 | 10826 | - | - | - | - | - | - | + |
| 29 | 10822 | - | - | + | - | + | - | + |
| 30 | 11881 | + | + | + | + | + | - | - |
| 31 | 10828 | - | - | + | - | + | - | + |
| 32 | 10824 | + | + | + | - | + | - | - |
| 33 | 10874 | - | - | - | - | - | - | + |
| 34 | 10827 | + | + | + | - | + | - | - |
| 35 | 11864 | - | - | - | - | - | - | + |
| 36 | 10820 | - | - | + | + | + | - | + |
| 37 | 11867 | + | + | + | + | - | - | + |
| 38 | 11882 | - | - | - | - | - | - | - |
| 39 | 10818 | + | + | + | - | - | - | - |
| 40 | 10821 | + | - | + | + | - | + | - |
| 41 | 10819 | + | + | - | + | + | + | + |
| 42 | 11879 | - | + | - | - | + | - | + |
| 43 | 11878 | - | + | + | - | - | + | + |
| 44 | 10814 | - | + | + | - | - | + | + |
| 45 | 11875 | - | - | - | - | - | - | - |
| 46 | 10730 | + | + | + | - | + | - | + |
| 47 | 10815 | + | + | + | - | + | + | + |
| 48 | 10816 | - | + | + | - | - | - | + |
| 49 | 10813 | - | + | + | - | - | - | + |
| 50 | 11877 | + | - | - | - | - | + | - |
| 51 | 10738 | - | - | + | - | - | + | - |
Table 3. Molecular markers banding pattern (+ for presence and - for the absence of bands).
The molecular markers screening for drought resistance also confirmed that the genotypes 11861, 11860, 10850, 10815, 10819, 11866, and 10825 had amplified the maximum number of drought resistance genes as 7, 7, 7, 6, 6, 6, and 6, respectively. The current study is in close agreement with previous results of (Kirigwi et al. 2007, Muhammad et al.2016; Muhammad et al., 2020; Khan et al.2016), who found the association of molecular markers with some quantitative traits in wheat genotypes. Therefore, these genotypes can better be adopted in drought habitats for high yield. All these genotypes can also be used in breeding programs to produce high-yielding varieties for drought-stress environments. The minimum number of drought-tolerant genes was found in 11868, 10853, 11873, 10826, 10874, and 11864 are considered to be drought susceptible.
Conclusion
The present research concluded that 11861, 11860, 10850, 10815, 10819, 11866, and 10825 have shown more resistant genes and are recommended for rainfed areas of Pakistan. The morphological parameters could be used for screening wheat germplasm for drought. Marker-assisted selection (MAS) is an advanced technique for screening drought tolerance. MAS is a cost-effective and more reliable technique and could be utilized in modern research.
Acknowledgment
We all authors are thankful to the Department of Genetics, the University of Hazara, for providing the facility to complete this project.
References
Ashraf, M. (2010). Inducing drought tolerance in plants: recent advances. Biotechnology Advances, 28:169-183. Blum, A. (1988). Plant breeding for stress environments. CRC Press.
Cao, Z., Deng, Z., Wang, M., Wang, X., Jing, J., Zhang, X., Li, Z. (2008). Inheritance and molecular mapping of an alien stripe-rust resistance gene from a wheat-Psathyrostachys huashanica translocation line. Plant Science, 174:544-549.
Khan, N., Ahmad, I., Inamullah, M., Muhammad, I., Zia, U., Ahmad, A., Khan, D., Khan, M., Razzaq, A. (2016). Morphogenetic screening of Pakistani spring wheat germplasm for drought tolerance. International Journal of Bioscience, 8:39-44.
Kirigwi, F.M., Van Ginkel, M.A.R.T.I.N., Brown-Guedira, G., Gill, B.S., Paulsen, G.M., Fritz, A.K. (2007). Markers associated with a QTL for grain yield in wheat under drought. Molecular Breeding, 20:401-413.
Mago, R., Spielmeyer, W., Lawrence, G., Lagudah, E., Ellis, J., Pryor, A. (2002). Identification and mapping of molecular markers linked to rust resistance genes located on chromosome 1RS of rye using wheat-rye translocation lines. Theoretical and Applied Genetics, 104:1317-1324.
Muhammad, I., Inamullah, H.A., Badshah Alam, I.A., Khan, I.A., Ziaullah, A.A., Najeebullah Khan, S.G.A. Marker assisted screening of wheat (Triticum aestivum L.) cultivars for drought tolerance and yield improvement. International Journal of Bioscience, 8:44-52. Muhammad, I., Li, K., Shuaib, M., Ayub, M., Shafqat, N., Alam, B., Azam, N. (2020). Identification of biotic stress tolerant wheat germplasm using morphological and molecular approaches. Gene Reports, 21:100928.
Naeem, I., Munir, I., Durrett, T.P., Iqbal, A., Aulakh, K.S., Ahmad, M.A., Hussain, F. (2020). Feasible regeneration and agro bacterium-mediated transformation of Brassica juncea with Euonymus alatus diacylglycerol acetyltransferase (EaDAcT) gene. Saudi Journal of Biological Sciences, 27:1324-1332.
Najafian, G. (2003). Screening of high volume breeding lines of hexaploid wheat for drought tolerance using cluster analysis based on kernel yield and STI. In Proceedings of 10th International Wheat Genetics Symposium, 2:1-6.
Passioura, J. (2007). The drought environment: physical, biological and agricultural perspectives. Journal of Experimental Botany, 58:113-117.
Plaut, Z., Butow, B.J., Blumenthal, C.S., Wrigley, C.W. (2004). Transport of dry matter into developing wheat kernels and its contribution to grain yield under post-anthesis water deficit and elevated temperature. Field Crops Research, 86:185-198. Rajki, E. (1982). Drought sensitive phase in development of wheat and possibility of testing drought resistance in the phytoron. Cereals Research Communication, 10:213-221.
Savin, R., Nicolas, M.E. (1999). Effects of timing of heat stress and drought on growth and quality of barley grains. Australian Journal of Agricultural Research, 50:357-364.
Shuaib, M., Bahadur, S., Hussain, F. (2020). Enumeration of genetic diversity of wild rice through phenotypic trait analysis. Gene Reports, 21:100797.
Stepien, L., Golka, L., Chelkowski, J. (2003). Leaf rust resistance genes of wheat: identification in cultivars and resistance sources. Journal of Applied Genetics, 44:139-149.
Weining, S., Langridge, P. (1992). Identification and mapping of polymorphism in cereals base on polymerase chain reaction. Theoritial Applied Genetics, 82:209-216.
Author Info
D. Khan1, I. Muhammad1, M. Shuaib2*, F. Hussain3, M. Romman4, N. Azam5, S. Abidullah6, A. Zeb6, A. Rauf6 and S. Bahadur72School of Ecology and Environmental Science, Yunnan University, Kunming, China
3Department of Microbiology, Cholistan University of Veterinary and Animal Sciences (CUVAS), Punjab, Bahawalpur 63100, Pakistan
4Department of Botany, University of Chitral, KPK, Pakistan
5Cenetre of Plant Biodiversity, University of Peshawar, Pakistan
6Department of Botany, Abdul Wali Khan University Madan, Pakistan
7Institute of Tropical Agriculture and Forestry, Hainan University, Haikou, China
Citation: Khan, D., Muhammad, I., Shuaib, M., Hussain, F., Romman, M., Azam, N., Abidullah, S., Zeb, A., Rauf, A., Bahadur, S. (2021). Investigation of grain yield and drought resistance in selected wheat lines on the basis of molecular markers. Ukrainian Journal of Ecology, 11 (5), 44-50.
Received: 15-Jun-2021 Accepted: 25-Jun-2021 Published: 30-Jul-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
