Research - (2022) Volume 12, Issue 8
LIPID PEROXIDATION AND ENZYME ACTIVITY IN SOYBEAN UNDER COMPLEX ACTION OF FUNGICIDAL SUBSTANCES AND BRADYRHIZOBIUM JAPONICUM
Т.P. Mamanko* and S. Ya KotsAbstract
The study of the peculiarities of biochemical processes that occur in the early stages of interaction of macro- and microsymbiotes is important to determine their participation in the formation of tolerance and realization of the symbiotic potential of established legume-rhizobial symbiosis under the influence of additional factors of various natures. The aim of the study was to investigate the effect of fungicidal substances in combination with nodule bacteria Bradyrhizobium japonicum on the accumulation of lipid peroxidation products, the activity of the key antioxidant enzyme ascorbate peroxidase and the effectiveness of nitrogenase enzyme complex in soybeans. Microbiological, physiological and biochemical research methods are used in the work. It was found that treatment of soybean seeds with fungicides in combination with inactive rhizobia induces a decrease in the content of lipid peroxidation products and an increase in ascorbate peroxidase activity in roots, which is accompanied by a slowing of nodulation processes in the early stages of ontogenesis. The use of fungicides together with active rhizobia, no significant changes in the accumulation of TBA-active products and increased activity of ascorbate peroxidase in soybean roots and nodules were recorded. This occurred along with the activation of the processes of nodulation and nitrogen fixation in soybean root nodules, formed by active rhizobia under the action of fungicides. It is concluded that the course of biochemical processes in the early stages of soybean-rhizobial symbiosis depends on the ability to realize the symbiotic properties of the strain Bradyrhizobium japonicum in combination with the active substances of fungicides.
Keywords
Glycine max (L.) Merr, TBA-active products, ascorbate peroxidase, nitrogenase activity, nodulation, rhizobia, symbiotic system, fungicide.
Introduction
When establishing a symbiotic interaction between symbiosis partners, specific signals are exchanged and a cascade of molecular events is induced, which lead to the activation of symbiotic pathways in the host plant and at the same time to the suppression of its protective systems (Wang Q, 2018). In the process of recognizing symbiosis partners involved on the one hand rhizobial lipohitooligosaccharides (Nod-factors), and on the other-receptor-like kinases (RLK) of legumes (Oldroyd GE, 2011). Nod factors are synthesized by rhizobia as a result of the expression of nod genes that initiate the synthesis of phenolic compounds secreted by the plant into the rhizosphere (Gourion B, 2015).
To date, it has been investigated that drugs with fungicidal activity cause disruption of the regulatory signaling system between macro- and microsymbionts by blocking the activity of nodulation genes and a decrease in the level of rhizobial Nod-factor (Bikrol A, 2005). In this case, each of the studied chemicals competitively limited the activation of gene Nod-factor depending on its concentration and inhibitory effect (Fox JE, 2007). It is known that legume plants with an active symbiotic apparatus are resistant to a wide range of diseases, and a clear combination of all measures aimed at optimizing the process of symbiosis contributes to the realization of the productive potential of plants (Joshi J, 2014). In this aspect, studies on the possibility of using fungicides to regulate the metabolism of legumes in symbiosis with nodula bacteria and increase the tolerance of symbiotic systems to biotic factors are relevant (Dicheng MD., 2018; Standish JR., 2018; Tackenberg M., 2018).
Our previous research has shown that the degree of influence of drugs with fungicidal action on the effectiveness of the formation and functioning of symbiotic systems of soybeans with nodule bacteria depends on the nature of the action of active substances in their composition (Kots SYa., 2018). Among the fungicides we studied were Standak Top (BASF, Germany) and Maxim XL (Syngenta, Switzerland), which caused different effects on the growth and development of soybean in symbiosis with rhizobia. This was manifested primarily in the induction of a shift in the nodulation ability of rhizobia and nitrogen-fixation activity of root nodules to later stages of ontogenesis-the formation of beans under the action of the fungicide drug Standak Top. At the same time, no such effect of influence on the realization of symbiotic properties of soybean plants under the action of the fungicidal drug Maxim XL was found (Kots SYa., 2018).
Standak Top contains a complex of chemical compounds (fipronil, 250 g/L, thiophanate methyl, 225 g/L, pyraclostrobin, 25 g/L), which combine fungicidal and insecticidal action. The action of fipronil [5-amino-[2,6-dichloro-4- (trifluoromethyl) phenyl]-4-[(1R, S)(trifluoromethyl)sulfinyl]-1H-pyrazole-3-carbonitrile] is to block gamma-aminobutyric acid, which regulates the passage of nerve impulses through chlorine channels in the membranes of insect nerve cells. Pyraclostrobin [Methyl N-{2-[1-(4-chlorophenyl)-1H-pyrazol-3-yloxymethyl] phenyl} (N-methoxy) carbamate] inhibits mitochondrial respiration by blocking electron transfer and disrupting energy metabolism in pathogen cells. Thiophanate-methyl [1,2-di-(3-methoxycarbonide-2-thiourido)-benzene] inhibits ergosterol production as well as nucleic acid biosynthesis in fungal cells [https://www.pesticidy.ru]. It is possible that the combination of such a complex of compounds in the preparation Standak Top is very physiologically active for soybeans, as a very sensitive crop to growing conditions.
Maxim XL contains two active substances (fludioxonil, 25 g/L, metalaxyl, 10 g/L). The mechanism of action of fludioxonil [4-(2,2-difluoro-1,3-benzodioxol-4-yl)-pyrrole-3-carboxylic acid] is associated with processes that induce disruption of membrane transport functions in the cells of the pathogen. Another substance in this drug-metalaxyl [N-(2,6-dimethylphenyl)-N-(2-methoxyacetyl) alanine methyl ether] acts by inhibiting the synthesis of all types of RNA, which leads to slowing and disruption of mitosis. It was studied that the active substances in the preparation of Maxim XL are absolutely safe and, killing pathogens, do not inhibit the beneficial soil microflora [https ://www.pesticidy.ru].
Invasion of rhizobia in the cells of the root hairs of legumes, similar to the process of pathogenesis, intensifies oxidative processes in plant cells, which is accompanied by increased generation of reactive oxygen species (ROS) (Murray JD., 2011). ROS can perform both signal and toxic functions under the action of various stressors on plants (Mhamdi A, 2018). There is a clear regulation of plant organism homeostasis, which includes a multifactorial system for ROS production and their detoxification (Hasanuzzaman M, 2020). The crucial role in maintaining the balance of these processes is played simultaneously by two systems of the plant organism-the antioxidant system for utilizing excess ROS and the signal system, which ensures the activation of defense mechanisms (Noctor G, 2018). An important role in the perception of signals is played by cell membranes, where most of the starting enzyme systems, which are involved in the initiation of calcium, lipoxygenase, phosphatide-oxalate signaling systems (Niki E., 2005). Plasmolemma also localizes enzymes that produce ROS, in particular NADP-oxidase, some forms of peroxidases (Zhu SY, 2018). Therefore, there is no doubt that the processes of lipid peroxidation (POL) are involved in the regulation of systems for maintaining homeostasis of plant cells, including the establishment of microbial-plant interactions.
Ascorbate peroxidase (APO) is one of the key enzymes of antioxidant protection of cells, which plays an important role in the regulation of ROS levels in plants due to external factors of various nature (ЕC 1.11.1.11) (Hasanuzzaman M, 2019). APO is involved in cross-transduction pathways with other antioxidant enzymes that utilize excess ROS and stabilize the internal biochemical state of the cell under the action of stressors, which leads to plant adaptation (Asthir B, 2020).
The study of the specificity of physiological and biochemical processes in legumes in symbiosis with nodule bacteria using fungicidal substances is necessary to find effective symbiotic systems that will be able to maximize the realization of nitrogen-fixation potential and at the same time have high tolerance to external factors.
The aim of the study was to investigate the effect of fungicidal substances in combination with nodule bacteria Bradyrhizobium japonicum on the accumulation of lipid peroxidation products, the activity of the key antioxidant enzyme ascorbate peroxidase and the effectiveness of nitrogenase enzyme complex, which fixes atmospheric molecular nitrogen in soybeans.
Materials and Methods
The objects of study were selected symbiotic systems formed with the participation of soybean plants (Glycine max (L.) Merr.) varieties Almaz and different strains of Bradyrhizobium japonicum 634b (active, virulent) and 604k (inactive, highly virulent) using fungicides Maxim XL 035 PS (Syngenta, Switzerland) and Standak Top (BASF, Germany). We used Bradyrhizobium japonicum strains from the museum collection of symbiotic nitrogen fixation department of the Institute of Plant Physiology and Genetics of the National Academy of Sciences of Ukraine.
Before sowing, soybeans were treated with solutions of fungicides, calculated on the basis of one rate of expenditure of the active substance of each preparation indicated by the producer per ton of seeds. One part of the seeds treated with fungicides was inoculated with rhizobia suspension for one hour. The other part of seeds treated with fungicides were sown without inoculated with rhizobia. A separate variant of the experiments was soybean seeds, which not treated with fungicides, but inoculated with nodule bacteria.
The culture of rhizobium was grown on solid mannitol-yeast medium for 9 days at 26-28°C (the titer of bacteria was 108 cells/mL). The inoculation load was 200-300 thousand rhizobia cells per seed. The control was non-inoculated plants, as well as inoculated with rhizobia without the use of fungicide seed treatment.
Plants were grown in sand pots in the application of a nutritional mixture of Hellrigel with 0.25 nitrogen rates from natural light and optimal water supply. For research, soybean roots were selected in the early stagesof ontogenesis-primordial leaves, first true leaf, second true leaf, and root nodules in the stageof third true leaf.
The nodulation ability of B. japonicum was determined by counting the mass of root nodules. The efficiency of molecular nitrogen fixation by soybean root nodules was evaluated by the activity of the nitrogenase (ЕC 1.18.6.1) enzyme complex, which was measured on gas chromatograph «Agilent GC system 6850» (USA) with flame-ionization detector (Hardy RWF, 1968). The separation of gases was carried out on a column (Supelco Porapak N) at a thermostat temperature of 55°C and a detector-150°C. The carrier gas was helium (20 ml per 1 minute). The volume of the analyzed sample of the gas mixture was 1 cm3. As a standard, pure ethylene (Sigma-Aldrich, No. 536164, USA) was used. The total nitrogenase activity was expressed in molar units of ethylene formed (μmol C2H4) per plant per hour. The specific value of the enzyme activity was calculated per unit mass of root nodules from one plant and expressed in molar units of ethylene formed per hour (μmol C2H4/g of nodules·h).
To obtain the enzyme extract, the weight of the plant material was homogenized with a cooled 60 mM phosphate buffer (pH 7.5) containing 2 mM ethylenediaminetetraacetic acid, 1 mM phenylmethylsulfonyl fluoride, 5 mM β-mercaptoethanol and 1% polyvinylpyrrolidone. The homogenate was centrifuged at 10,000 rpm for 20 minutes at 4°C. The supernatant was used to determine the APO enzyme activity using the UV-1900 scanning two-beam spectrophotometer (Shimadzu, Japan).
APO activity was measured by a decrease in optical density at a wavelength of 290 nm for 2 min as a result of oxidation of ascorbate (ɛ=2.8 mm-1 cm-1) (Nakano Y, 1981). The reaction mixture contained 60 mm potassium phosphate buffer (pH 7.0), 0.1 mm ethylenediaminetetraacetic acid, 0.2 mm ascorbate, 0.1 mm hydrogen peroxide. The reaction was initiated by the addition of supernatant. The results are presented in μmols of oxidized ascorbate in terms of the content (mg) of total soluble protein per minute, which was determined by Bradford (Bradford M, 1976).
The intensity of lipid peroxidation was assessed by the content of TBA-active products, determined as a result of a color reaction with thiobarbituric acid (TBA) (Heath R, 1968). To obtain a plant extract, samples of the test material (0.5 g) are homogenized with 3 ml of distilled water. To the homogenate add 3 ml of trichloroacetic acid and homogenize a second time. Two samples of 2 ml each are taken from the resulting homogenate. To one of them add 2 ml of trichloroacetic acid, which is then used as a control on a spectrophotometer, and to the other-2 ml of a solution of thiobarbituric acid. The samples were incubated for 30 min in a boiling water bath, cooled and centrifuged at 3000 rpm for 10 min. The supernatant was collected by syringe in test tubes and measured on a spectrophotometer at λ=532 nm. The values of non-specific absorption were calculated at λ=600 nm. The results are presented in nanomoles of MDA per gram of wet weight using a molar extinction coefficient 1.55 × 105 cm-1ꞏM-1.
The results were statistically analyzed in the Statistica 6.0 (Statsoft Inc., USA) program pack. The tables and figures show the arithmetic mean values and their standard errors (x ± SE). The reliability of the differences between the samples was evaluated using the single-factor dispersion analysis (ANOVA). Differences were considered to be significant at P<0.05.
Results and Discussion
It was found that the treatment of soybean seeds with the fungicide Standak Top leads to an increase in the content of TBA-active products in the roots by 16.1% in the stage of primordial leaves, compared with untreated plants (Fig. 1А). At the same time, in this stage of ontogenesis, no significant differences in lipid peroxidation in soybean roots were found between the variants of the experiment with seed treatment with Maxim XL fungicide and without its use. In the stage of the first true leaf, intensive development of lipid peroxidation in soybean roots was observed under the action of the fungicide Standak Top, as evidenced by the increase in the content of TBA-active products by 53.2%. Whereas under the action of Maxim XL, their level did not increase significantly in the roots by 14.8%, compared with untreated plants. In the stage of the second true leaf, the level of lipid peroxidation products slightly increased in soybean roots under the action of both fungicides by 16.1% (Maxim XL) and 11.3% (Standak Top), compared to untreated plants.
Inoculation of soybean seeds with inactive, highly virulent strain of rhizobia 604k led to a decrease in the content of TBA-active products in the roots by 16.1% in the stage of primordial leaves and increase their content in the stages of the first and second true leaves by 27.2 and 30.3%, in comparison with non-inoculated plants (Fig. 1А and 1В). The use of soybean seed treatment with Maxim XL fungicide together with rhizobia of inactive strain 604k did not lead to significant changes in lipid peroxidation in soybean roots in the stage of primordial leaves and the first true leaf, in comparison with inoculated plants without fungicide (Fig. 1В). In the stage of the second true leaf, the development of these processes in soybean roots decreased by 17.3% under the action of the fungicide Maxim XL in an inefficient symbiotic system. During the treatment of soybean seeds with the fungicide Standak Top together with rhizobia of the inactive strain 604k, a decrease in the intensity of lipid peroxidation in the roots in the early stages of plant ontogenesis was recorded (Fig. 1В). This was especially evident in the stage of primordial leaves, when the content of TBA-active products in soybean roots decreased by 25.8%, while in the stages of the first and second true leaves, decreased by 11.1 and 21.9%, respectively, compared with inoculated plants without the use of fungicide treatment.
Inoculation of soybean seeds with the active virulent strain rhizobia 634b induced a significant decrease in the content of TBA-active products in the roots by 38.3% in the stage of primordial leaves, compared with non-inoculated plants (Fig. 1А and 1С). In the following stages of ontogenesis before the formation of the second true leaf, no significant differences were recorded in the processes of lipid peroxidation in soybean roots between variants with inoculation of seeds with rhizobia of active strain 634b and without its use.
The use of pre-sowing treatment of soybean seeds with fungicide Maxim XL together with rhizobia of active strain 634b led to a decrease in the concentration of TBA-active products in the roots in the stage of primordial leaves, the content of which was even 22.5% lower than in inoculated plants without fungicide (Fig. 1С). In the subsequent stages of ontogenesis, to the formation of the third true leaf, significant differences in the course of lipid peroxidation in soybean roots were not observed by treatment of rhizobia with fungicide Maxim XL, as compared to inoculated plants without fungicide.
In the effective symbiotic system under the action of the fungicide Standak Top no significant differences were found in the course of lipid peroxidation in soybean roots in the stage of primordial leaves, in comparison with inoculated plants without the use of fungicide treatment (Fig. 1С). While in the stage of the first true leaf, the concentration of TBA-active products in the roots of soybeans inoculated with rhizobia together with the fungicide Standak Top increased by 15.8%. In the stage of the second true leaf, their content almost reached the level of inoculated plants without the use of fungicide treatment.
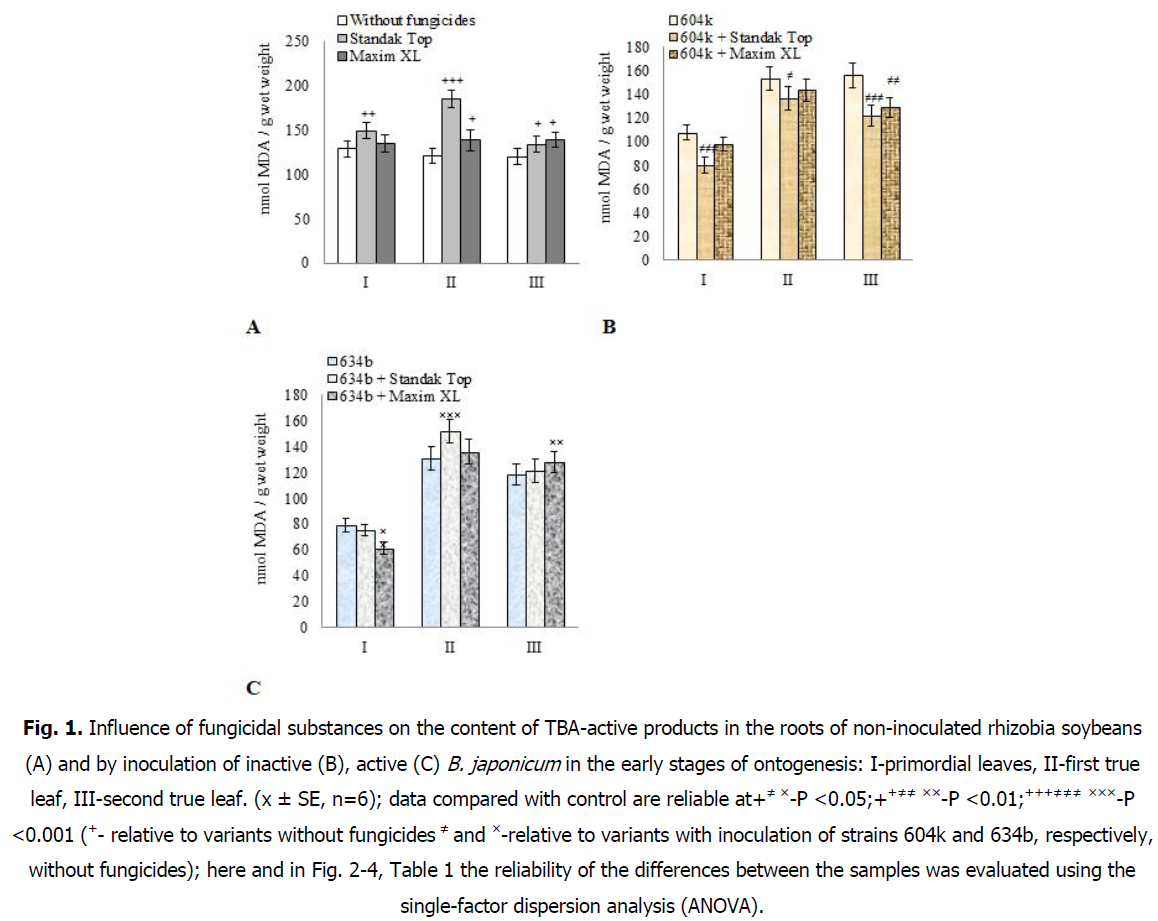
Fig 1: Influence of fungicidal substances on the content of TBA-active products in the roots of non-inoculated rhizobia soybeans (A) and by inoculation of inactive (B), active (C) B. japonicum in the early stages of ontogenesis: I-primordial leaves, II-first true leaf, III-second true leaf. (x ± SE, n=6); data compared with control are reliable at+≠ ×-P <0.05;++≠≠ ××-P <0.01;+++≠≠≠ ×××-P <0.001 (+- relative to variants without fungicides ≠ and ×-relative to variants with inoculation of strains 604k and 634b, respectively, without fungicides); here and in Fig. 2-4, Table 1 the reliability of the differences between the samples was evaluated using the single-factor dispersion analysis (ANOVA).
It is shown that the treatment of seeds with substances with fungicidal activity led to an increase in the activity of APO in soybean roots, regardless of the nature of the action of active substances (Fig. 2A). In particular, during the early stages of ontogenesis, primordial leaves, first and second true leaves, the activity of the enzyme in soybean roots under the action of Standak Top increased by 46.8, 20.7 and 25.7%, respectively, while with the use of Maxim XL by 39,9, 38,2, 42,8%, in comparison with control plants without use of fungicides.
When inoculating soybean seeds with an inactive highly virulent strain of rhizobia 604k, an increase in APO activity in soybean roots by 31.7% in the stage of primordial leaves and by 24.0% in subsequent stages of ontogenesis was observed, compared with non-inoculated plants (Fig. 2B).
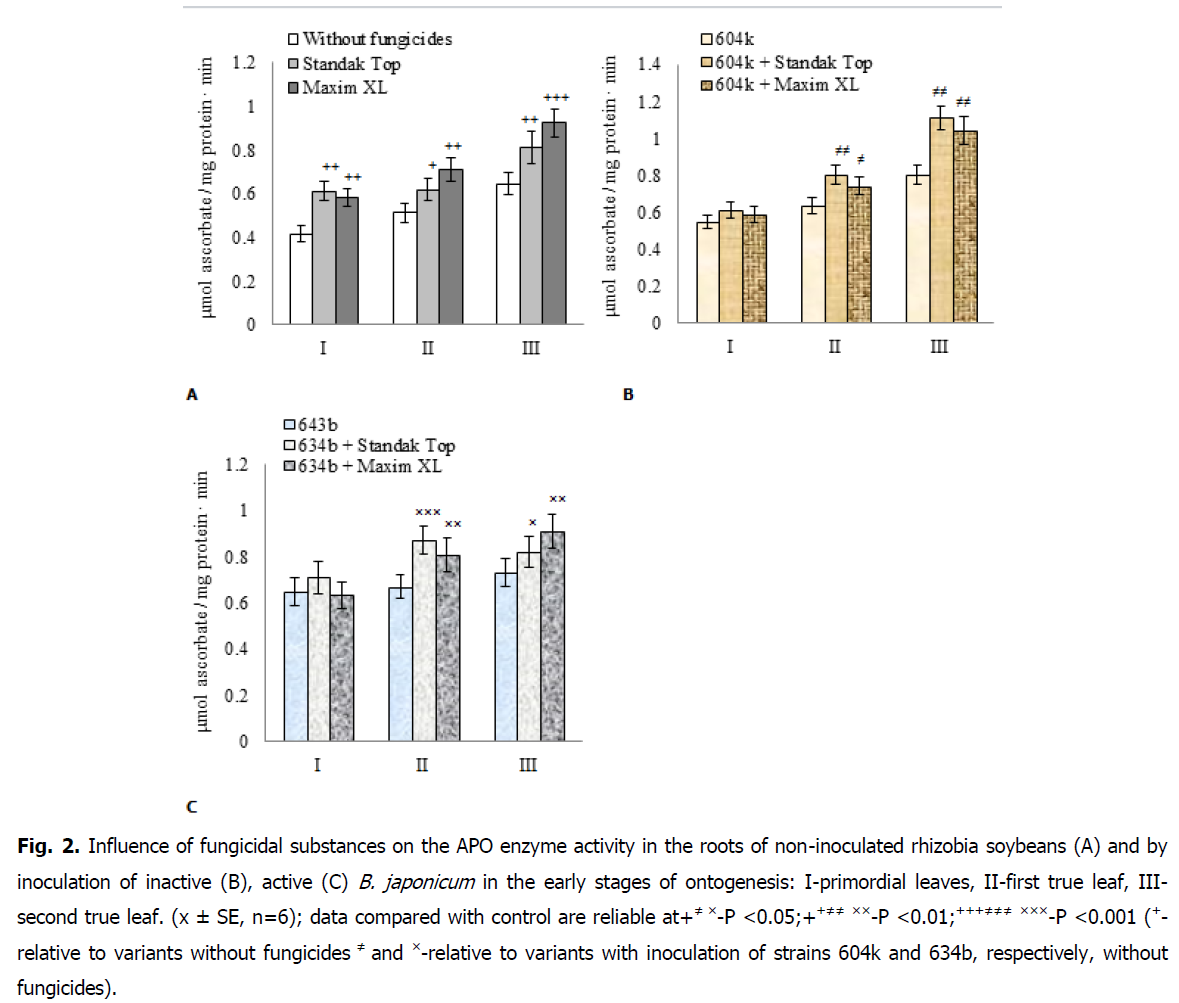
Fig 2: Influence of fungicidal substances on the APO enzyme activity in the roots of non-inoculated rhizobia soybeans (A) and by inoculation of inactive (B), active (C) B. japonicum in the early stages of ontogenesis: I-primordial leaves, II-first true leaf, III-second true leaf. (x ± SE, n=6); data compared with control are reliable at+≠ ×-P <0.05;++≠≠ ××-P <0.01;+++≠≠≠ ×××-P <0.001 (+- relative to variants without fungicides ≠ and ×-relative to variants with inoculation of strains 604k and 634b, respectively, without fungicides).
Treatment of soybean seeds with substances with fungicidal activity in combination with inoculation with inactive rhizobia did not lead to significant changes in APO activity in the roots, compared with inoculated plants without the use of fungicides in the stage of primordial leaves.
In subsequent stage of ontogenesis, the enzyme activity increased in soybean roots under the combined action of rhizobia and fungicides. Thus, for the use of the fungicide Standak Top by 26.3 and 38.4%, and under the influence of Maxim XL by 16.8 and 30.0%, respectively, in the stages of the first and second true leaves.
Inoculation of soybean seeds with active virulent strain rhizobia 634b revealed an increase in APO activity in soybean roots in the stage of primordial leaves and the first true leaf, respectively, by 56.2 and 30.8%, while in the stage of the second true leaf recorded a significant increase in activity enzyme by 13.0%, compared with non-inoculated plants (Fig. 2C).
Treatment of soybean seeds by fungicidal substances in combination with active rhizobium did not induce a change in the activity of APO in the roots in the stage of primordial leaves, compared to inoculated plants without fungicide treatment. In the stage of the first true leaf, the activity of the enzyme in soybean roots increased by 29.8% under the combined action of rhizobia and Standak Top, and by 20.3% when using rhizobia together with Maxim XL. In the stage of the second true leaf, an increase in the enzyme activity in soybean roots was also recorded due to the action of these fungicides simultaneously with active rhizobia by 12.3% (Standak Top) and 24.6% (Maxim XL).
It was proved that in the stage of the third true leaf, the content of TBA-active products in the root nodules of soybeans inoculated with the inactive strain of rhizobia 604k was 2.1 times higher than in the root nodules of soybeans inoculated with the active strain of rhizobia 634b (Table 1). At the same time, in the root nodules of soybeans inoculated with inactive rhizobia, the APO activity was 3.3 times higher than the level of enzyme activity in soybeans inoculated with active rhizobia.
The application of soybean seed treatment with Standak Top fungicide led to a significant accumulation of lipid peroxidation products in soybean root nodules against the background of inoculation with rhizobia of different efficiency in the stage of three true leaves (Table 1). In particular, under the combined effect of Standak Top and inactive strain of rhizobia 604k, their content in root nodules increased by 26.4%, and under the simultaneous effect of this fungicide with rhizobia of active strain 634b-only 12.3%, compared with inoculated plants without the use of fungicides. When seed treatment with fungicide Maxim XL simultaneously with rhizobia of different efficiency, no significant differences were observed in the accumulation of lipoperoxidation products in soybean root nodules, in comparison with inoculated plants without the use of fungicides. When fungicidal substances were used simultaneously with inactive rhizobia of strain 604k, the activity of APO in root nodules was almost at the same level as in inoculated plants without of fungicides. Whereas under the influence of fungicides with active rhizobia strain 634b, an increase in the activity of the enzyme in the root nodules was observed by 40.3% (Standak Top) and 24.7% (Maxim XL), compared with inoculated rhizobia without of fungicides.
| Variant | nmol MDA/ g wet weight |
μmol ascorbate/ mg of protein ꞏ min |
|---|---|---|
| Strain 604k | 222.45 ± 15.25 | 0.621 ± 0.051 |
| Standak Top+strain 604k | 281.22 ± 19.14≠ | 0.674 ± 0.044 |
| Maxim XL+strain 604k | 236.74 ± 16.11 | 0.533 ± 0.032 |
| Strain 634b | 104.02 ± 7.18 | 0.186 ± 0.012 |
| Standak Top+strain 634b | 116.83 ± 8.11 | 0.261 ± 0.018×× |
| Маxim XL+strain 634b | 106.73 ± 9.12 | 0.232 ± 0.016× |
Table 1. Influence of fungicidal substances on the content of TBA-active products and APO enzyme activity in root nodules of soybean inoculated with different strains of B. japonicum. Stage of third true leaves. (x ± SE, n=6)
It was studied that under the action of fungicides together with rhizobia there is a slowing down of nodulation processes in the stage of the first and second true leaf (Fig. 3A and 3B). Thus, under the action of Standak Top in combination with rhizobia of inactive highly virulent strain 604k the mass of nodules decreased by 31.2-32.8%, and under the action of Maxim XL-18.7-35.1%, compared with inoculated plants without action of fungicides. Whereas under the influence of fungicides together with the active virulent strain of rhizobia 634b revealed almost the same mass of nodules on the roots as in inoculated soybeans without fungicides.
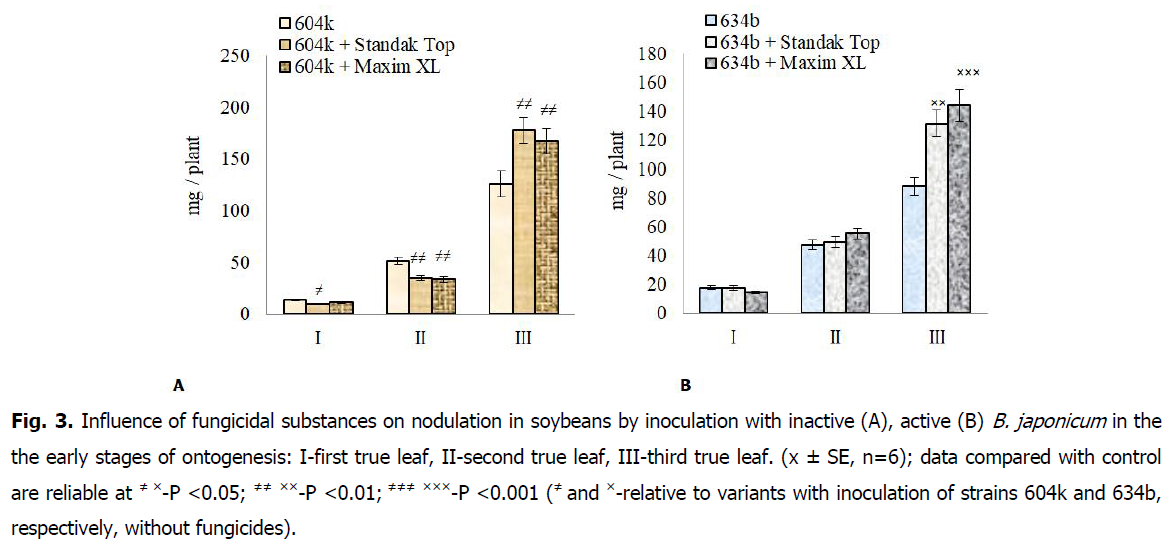
Fig 3: Influence of fungicidal substances on nodulation in soybeans by inoculation with inactive (A), active (B) B. japonicum in the the early stages of ontogenesis: I-first true leaf, II-second true leaf, III-third true leaf. (x ± SE, n=6); data compared with control are reliable at ≠ ×-P <0.05; ≠≠ ××-P <0.01; ≠≠≠ ×××-P <0.001 (≠ and ×-relative to variants with inoculation of strains 604k and 634b, respectively, without fungicides).
In the stage of the third true leaf, the nodulation on soybean roots are intensified in both symbiotic systems under the action of fungicidal drugs. In particular, under the action of Standak Top and Maxim XL together with inactive rhizobia of strain 604k the mass of nodules increases, respectively, by 41.3 and 32.5%, and together with active rhizobia of strain 634b-by 48.9 and 63.1%, compared with inoculated rhizobia without fungicides.
The use of treatment of soybean seeds with fungicides in combination with rhizobia of active strain 634b contributed to an increase the fixation of molecular nitrogen of the atmosphere by root nodules in the stage of the third true leaf. In particular, under the action of the fungicide Standak Top, the total activity of nitrogenase in the root nodules increased 1.7 times, while its specific value increased 1.1 times, compared with inoculated plants without the use of fungicides. With the use of the fungicide Maxim XL together with rhizobia of the active strain 634b, an increase in the total activity of the nitrogenase complex in the root nodules was observed 2.9 times, and the specific value of the enzyme increased 1.9 times (Fig. 4A and 4B).
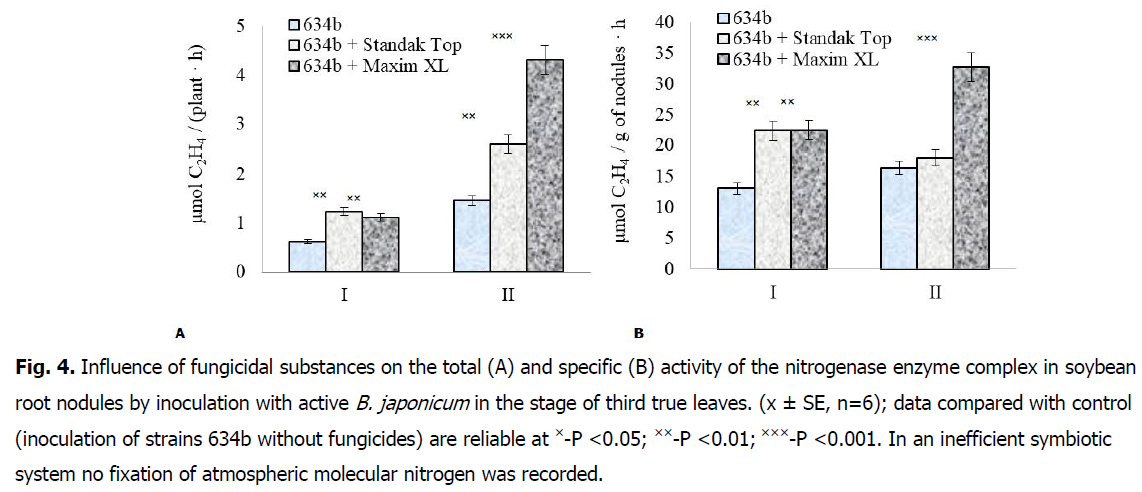
Fig 4: Influence of fungicidal substances on the total (A) and specific (B) activity of the nitrogenase enzyme complex in soybean root nodules by inoculation with active B. japonicum in the stage of third true leaves. (x ± SE, n=6); data compared with control (inoculation of strains 634b without fungicides) are reliable at ×-P <0.05; ××-P <0.01; ×××-P <0.001. In an inefficient symbiotic system no fixation of atmospheric molecular nitrogen was recorded.
The analysis of the obtained results showed that the action of fungicides induces the development of POL and intensifies the activity of APO in soybean roots, which was not inoculated with rhizobia. The tendency to change these biochemical parameters under the action of fungicides was the same during the early stages of soybean ontogenesis. However, under the action of Standak Top there was a significant accumulation of lipid peroxidation products. While under the action of Maxim XL a significant increase in APO activity in soybean roots was recorded. Such a different effect of fungicides on the course of the studied biochemical processes in soybean plants is obviously related to the nature of the action of active substances in their composition, as mentioned above.
Inoculation of soybean seeds with different efficiency of rhizobia leads to a decrease in the content of TBA-active products and an increase in the activity of APO in the roots in the stage of primordial leaves, compared with non-inoculated plants. The stage of primordial leaves is especially important for soybean plants in symbiosis with nodule bacteria. During this period of ontogenesis there is an active formation of symbiotic structures-the laying of nodular primordia. Probably, in this period of ontogenesis there are significant changes in the metabolism of soybean plants, which cause the corresponding changes in the course of the studied biochemical processes.
In the stages of the first and second true leaves in soybeans in symbiosis with the inactive rhizobia strain 604k, an increase in the content of TBA-active products and the level of APO activity in the roots and root nodules was observed in comparison with the active symbiosis formed with the active rhizobia strain 634b.
In the stages of the first and second true leaves, there is an intensive growth of nodules on soybean roots-nodulation processes are activated. At the same time, in the root nodules, although very slow, begin the processes associated with the fixation of molecular nitrogen in the atmosphere. An inefficient symbiotic system formed with the participation of the rhizobia strain 604k forms a large number of nodules on soybean roots, which cannot perform the nitrogen-fixation function and fully realize their symbiotic potential. This can induce changes in the course of biochemical processes, including POL and activity of antioxidant defense systems, as an appropriate response to the formation of stress-protective state of plants to the insufficient needs of the plant in nitrogen.
The use of fungicides in an ineffective symbiotic system formed with the inactive strain of rhizobia 604k led to a decrease in the content of lipid peroxidation products in soybean roots, compared with inoculated plants without fungicides, during the early stages of ontogenesis. Whereas under the action of fungicides and inactive rhizobia there was no change in the level of APO activity in soybean roots in the stage of primordial leaves and a significant increase in enzyme activity in subsequent stages of ontogenesis. Under the influence of fungicides together with the active rhizobia of strain 634b, no significant changes were observed in the accumulation of lipid peroxidation products and increased APO activity in the roots compared to inoculated plants without fungicides.
Earlier, we found changes in metabolic processes in soybeans in symbiosis with nodule bacteria, in response to the action of fungicides, which was accompanied by a slowdown in plant development in the early stages of ontogenesis. At the same time, this was accompanied by adaptive reorganizations of plant metabolism in the formed symbioses and did not negatively affect their development in the subsequent stages of ontogenesis (Mamenko TP, 2019).
It was found that the action of fungicides in combination with rhizobia induced a decrease in the mass of nodules on soybean roots in the stage of the first and second true leaves. Such suppression of nodulation in soybeans was especially evident when the fungicides were combined with an inactive rhizobia strain. However, in the stage of third true leaves, an increase in the nodule formation on soybean roots was observed under the combined action of fungicides and rhizobia of different efficiency.
In the stage of the third true leaf, along with the intensification of nodulation processes, the root nodules actively perform their nitrogen-fixation function. In the presented studies we showed the activity of the nitrogenase complex in the root nodules formed by the active strain of rhizobia 643b in the stage of active nitrogen fixation-the third true leaf, in the absence of this function in ineffective symbiosis with strain 604k. Under the action of rhizobia in combined with fungicides, the activity of the enzyme nitrogenase complex increases in the root nodules of soybeans, especially under the action of Maxim XL. The obtained positive effect from the treatment of seeds by rhizobia with fungicides on the efficiency of nitrogen uptake by soybean plants is proved by the values of nitrogenase enzyme activity in terms of whole plant weight (total value) and unit weight of nodules (specific value). Similar dynamics of increasing the activity of the enzyme nitrogenesis complex in soybeans using the combined treatment of seeds with rhizobia with fungicides we have shown in previous works (Mamenko TP, 2019), as this is the most important indicator of assessing the effectiveness of the symbiotic apparatus in formed legume-rhizobial symbioses.
In the stage of the third true leaf, along with the intensification of nodulation processes, the root nodules actively perform their nitrogen-fixation function. In the presented studies we showed the activity of the nitrogenase complex in the root nodules formed by the active strain of rhizobia 643b in the stage of active nitrogen fixation-the third true leaf, in the absence of this function in ineffective symbiosis with strain 604k. Under the action of rhizobia in combined with fungicides, the activity of the enzyme nitrogenase complex increases in the root nodules of soybeans, especially under the action of Maxim XL. The obtained positive effect from the treatment of seeds by rhizobia with fungicides on the efficiency of nitrogen uptake by soybean plants is proved by the values of nitrogenase enzyme activity in terms of whole plant weight (total value) and unit weight of nodules (specific value). Similar dynamics of increasing the activity of the enzyme nitrogenesis complex in soybeans using the combined treatment of seeds with rhizobia with fungicides we have shown in previous works (Mamenko TP, 2019), as this is the most important indicator of assessing the effectiveness of the symbiotic apparatus in formed legume-rhizobial symbioses.
Thus, differences in the processes of lipid peroxidation and activity of the antioxidant enzyme APO in soybean roots and nodules under the action of rhizobia together with fungicidal substances indicate the specificity of the complex of biochemical processes that determine the formation and functioning of soybean-rhizobial symbiosis. However, despite the specificity of these processes in soybean plants in symbiosis with Bradyrhizobium japonicum for the use of fungicides, they induced adaptive changes in metabolism, which were accompanied by an increase in nitrogen fixation processes to supply the plant's nitrogen needs. And the recorded increase in the efficiency of the symbiotic apparatus of soybeans indicates the possibility of mitigating the toxic effects of the active substances of fungicides on plant metabolism through combined use with active rhizobia.
Conclusion
Treatment of soybean seeds with fungicides in combination with inactive rhizobia induces a decrease in the content of lipid peroxidation products and an increase in APO activity in roots, which is accompanied by a slowing of nodulation processes in the early stages of ontogenesis.
The use of fungicides together with active rhizobia, no significant changes in the accumulation of TBA-active products and increased activity of APO in soybean roots and nodules were recorded. This occurred along with the activation of the processes of nodulation and nitrogen fixation in soybean root nodules.
The course of physiological and biochemical processes in the early stages of soybean-rhizobial symbiosis depends on the ability to realize the symbiotic properties of the strain Bradyrhizobium japonicum in combination with the active substances of fungicides.
References
Wang, Q., Liu, J., Zhu, H. (2018). Genetic and molecular mechanisms underlying symbiotic specificity in legume-rhizobium interactions. Frontiers in Plant Science, 9:313.
Oldroyd, G.E., Murray, J.D., Poole, P.S., Downie, J.A. (2011). The rules of engagement in the legume-rhizobial symbiosis. Annual Review of Genetics, 45:119-144.
Gourion, B., Berrabah, F., Ratet, P., Stacey, G. (2015). Rhizobium-legume symbioses: the crucial role of plant immunity. Trends in Plant Science, 20:186-194.
Bikrol, A., Saxena, N., Singh, K. (2005). Response of Glycine max in relation to nitrogen fixation as influenced by fungicide seed treatment. African Journal of Biotechnology, 4:667-671.
Fox, J.E., Gulledge, J., Engelhaupt, E., Burow, M.E., McLachlan, J.A. (2007). Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants. Proceedings of the National Academy of Sciences, 104:10282-10287.
Joshi, J., Sharma, S., Guruprasad, K.N. (2014). Foliar application of pyraclostrobin fungicide enhances the growth, rhizobial-nodule formation and nitrogenase activity in soybean (var. JS-335). Pesticide Biochemistry and Physiology, 114:61-66.
Ma, D., Zhu, J., He, L., Cui, K., Mu, W., Liu, F. (2018). Baseline sensitivity of Phytophthora capsici to the strobilurin fungicide benzothiostrobin and the efficacy of this fungicide. European Journal of Plant Pathology, 152:723-733.
Standish, J.R., Brenneman, T.B., Stevenson, K.L. (2018). Dynamics of fungicide sensitivity in Venturia effusa and fungicide efficacy under field conditions. Plant Disease, 102:1606-1611.
Tackenberg, M., Volkmar, C., Schirrmann, M., Giebel, A., Dammer, K.H. (2018). Impact of sensor‐controlled variable‐rate fungicide application on yield, senescence and disease occurrence in winter wheat fields. Pest Management Science, 74:1251-1258.
Kots, S.Y., Mamenko, T.P., Pavlyshche, A.V. (2018). Activity of nitrogen fixation and antioxidant enzymes in symbiotic systems Glycine max–Bradyrhizobium japonicum for complex treatment with lectin and fungicides. Regulatory Mechanisms in Biosystems, 9:148-155.
Murray, J.D. (2011). Invasion by invitation: rhizobial infection in legumes. Molecular Plant-Microbe Interactions, 24:631-639.
Mhamdi, A., Van Breusegem, F. (2018). Reactive oxygen species in plant development. Development, 145:164376.
Hasanuzzaman, M., Bhuyan, M.B., Zulfiqar, F., Raza, A., Mohsin, S.M., Mahmud, J.A., Fotopoulos, V. (2020). Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants, 9:681.
Noctor, G., Reichheld, J.P., Foyer, C.H. (2018). ROS-related redox regulation and signaling in plants. In Seminars in Cell and Developmental Biology, 80:3-12.
Niki, E., Yoshida, Y., Saito, Y., Noguchi, N. (2005). Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochemical and Biophysical Research Communications, 338:668-676.
Zhu, S.Y., Zhuang, J.S., Wu, Q., Liu, Z.Y., Liao, C.R., Luo, S.G., Zhong, Z.M. (2018). Advanced oxidation protein products induce pre‐osteoblast apoptosis through a nicotinamide adenine dinucleotide phosphate oxidase‐dependent, mitogen‐activated protein kinases‐mediated intrinsic apoptosis pathway. Aging Cell, 17:e12764.
Hasanuzzaman, M., Bhuyan, M.B., Anee, T.I., Parvin, K., Nahar, K., Mahmud, J.A., Fujita, M. (2019). Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants, 8:384.
Asthir, B., Kaur, G., Kaur, B. (2020). Convergence of pathways towards ascorbate–glutathione for stress mitigation. Journal of Plant Biology, 63:243-257.
Hardy, R.W., Holsten, R.D., Jackson, E.K., Burns, R. (1968). The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiology, 43:1185-1207.
Nakano, Y., Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22:867-880.
Bradford, M. (1976). Rapid and sensitive method for the quantization of the microgram quantities of protein utilizing: the principle of protein-dye binding. Analytical Biochemistry, 72:248-254.
Heath, R.L., Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125:189-198.
Mamenko, T.P., Khomenko, Y.O., Kots, S.Y. (2019). Influence of fungicides on activities of enzymes of phenolic metabolism in the early stages of formation and functioning of soybean symbiotic apparatus. Regulatory Mechanisms in Biosystems, 10:111-116.
Author Info
Т.P. Mamanko* and S. Ya KotsCitation: Mamanko, Т.P., Kots, S.Ya. (2022). Lipid Peroxidation and Enzyme Activity in Soybean Under Complex Action of Fungicidal Substances and Bradyrhizobium japonicum. Ukrainian Journal of Ecology. 12:20-30.
Received: 15-Mar-2021, Manuscript No. UJE-21-27833; , Pre QC No. P-27833; Editor assigned: 17-Mar-2022, Pre QC No. P-27833; Reviewed: 28-Mar-2022, QC No. Q-27833; Revised: 22-Sep-2022, Manuscript No. R-27833; Published: 30-Sep-2022, DOI: 10.15421/2022_395
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
