Research Article - (2021) Volume 11, Issue 2
Abstract
The article presents the study's results of the morphogen development features of the Shrub roses explants during in vitro reproduction — five cultivars from the collection fund of the National Dendrological Park "Sofiyivka" of the National Academy of Sciences of Ukraine: 'Gärtnerfreude' (W. Kordes' Söhne, 1991), 'Lavender Dream' (G. P. Ilsink, 1984), 'Pomponella' (W. Kordes' Söhne, 2005), 'Red Cascade' (R. S. Moore, 1976), 'Sommerabend' (W. Kordes' Söhne, 1995). The listed cultivars belong to those Shrub roses that form a dense cover of shoots, leaves, and flowers and are applicable for decorating plots that are of little avail for growing other ornamental plants. Shrub roses of most varieties introduced into the National Dendrological Park "Sofiyivka" National Academy of Science of Ukraine have shown sufficient adaptive ability in the natural and climatic conditions of the introduction area. Studies of gas resistance have shown that roses of this class adapt well to the conditions of the high content of traffic fumes and dust while maintaining high decorative qualities, which gave reason to recommend them for wide use in landscaping settlements of the Right-Bank Forest-Steppe Zone of Ukraine. However, the experience of introducing Shrub roses into gardening has shown that to create landscape expositions, it is often necessary to have a large amount of planting material of a specific cultivar, and to solve the problem requires the use of new, promising propagation methods. One of them is the in vitro plant propagation method, developed based on biotechnology to preserve plants genetically identical to the original maternal form, which contributes to the production of morphologically aligned, genetically homogeneous planting material. Therefore, the study of the reproduction features of the garden class Shrub roses, cultivated in vitro, defined the dependence of the explants morphogen development on changes in the concentrations of cytokinins and auxins included in the nutrient media are relevant and have both scientific and practical interest. The authors revealed that the studied cultivars were successfully introduced into the culture in vitro, are capable of direct morphogenesis when using exogenous phytohormones 6-BAP, β-IBA, β-IAA, 1-NAA; however, they had unequal indicators in the morphogenetic reactions display the degree. It was found that the phytohormonal regulation of morphogen processes in explants was determined by the presence of specific ratios of both auxins and cytokinins in the nutrient medium. Using segments of shoots with axillary and apical buds, the authors obtained morphogen structures that were formed directly on explants by the direct morphogenesis type. Of the tested inducers of morphogenesis, the most effective for the Shrub roses was MS 6-BAP – 2.0 mg/l and β-IBA – 0.01 mg/l to the nutrient medium. Within 28–34 days after passage on nutrient media modified with the addition of phytohormones, active formation of morphogen structures was observed, which gave rise to shoots, the number of which averaged from 2.92 to 12.04 pcs. per explant. 'Red Cascade' and 'Sommerabend' cvs differed in the maximum indices of adventive shoot formation, which, in six passages, reached a multiplication rate of 12.2±0.33 and 7.24±0.35, respectively. ‘Gärtnerfreude’, ‘Lavender Dream’, ‘Pomponella’ cvs. under the influence of various concentrations of phytohormones had slightly lower indices of morphogen potential with the multiplication rate in the range of 3.66±0.15 to 6.33±0.11. Thus, the differentiation of meristemic tissues of the studied Shrub rose cultivars with the subsequent induction of morphogenesis depends on the concentration of both cytokinins and auxins in nutrient media and determines the successful reproduction of these plants in vitro. A short reproduction cycle allows for a short time to obtain the required amount of planting material while maintaining the necessary economically valuable traits.
Keywords
micropropagation, explants, nutrient medium, phytohormones, morphogenesis, multiplication rate.
Introduction
The garden class of Shrub roses, distinguished in the world rose classification in 1965, is undoubtedly considered to be the richest in variety of bushes, inflorescences, and shape of flowers (Cairns, 2003; Bumbeeva, 2010). Many of them are characterized by solid growth and repeat flowering since a significant part of the Shrub rose cultivars were obtained due to crossing Hybrid Tea roses, Floribunda, and other garden classes with climbing ones. Like climbing roses, long shoots reach 2–3 m long, and relatively large flowers and repeat-flowering are inherited from modern garden roses (Mattock, 1996, Beals, 1997).
Among the Shrub roses, ground-cover cultivars occupy a special place. They have spreading or outstretched bushes, the diameter of which is greater than the height. They form a dense cover of shoots, leaves, and flowers. Ground cover roses can be used to decorate plots that are little available for growing other ornamental plants – slopes, rocky hills (Jakobi, 2002).
As the experience of introducing the Shrub roses into the National Dendrological Park "Sofiyivka," National Academy of Sciences of Ukraine has shown, the roses of most cultivars have displayed sufficient adaptive ability in the natural and climatic conditions of the introduction area. The study of gas resistance demonstrated that the roses of this class adapt well to the conditions of the high content of traffic fumes and dust while maintaining high decorative qualities, which made it possible to recommend them for wide use in landscaping settlements of the Right-Bank Forest-Steppe of Ukraine (Moroz & Denysko, 2010; Moroz et al., 2012; Moroz & Denysko, 2014).
Simultaneously, the experience of introducing semi-climbing roses has shown that it is often necessary to have a significant amount of planting material (sometimes several thousand seedlings) of a specific variety to implement projects of landscape expositions. Therefore, obtaining mass planting material with the preservation of the genetic homogeneity of ornamental garden forms and varietal characteristics of plants requires the use of new, promising methods of reproduction.
One of the modern biotechnological methods is the reproduction of plants in vitro, developed to preserve the gene pool of valuable plant species genetically identical to the original maternal forms. This method helps to obtain and preserve morphologically aligned planting material and enables propagation plants with complicated seed or vegetative propagation, contributes to the improvement of the planting material, and speeds up its production several times (Valikhanova, 1996; Mel'nychuk et al., 2003; Kushnir & Sarnats'ka, 2005; Koval'chuk et al., 2008; Matskevych et al., 2010).
The ability of plants to differentiate cells, morphogenesis, and formation of a whole plant depend on the plant's species, genotype, specific tissue, and cell type. Different species and genotypes within a species and different types of plant cells have different abilities to regenerate. In most cases, a technique developed for a particular genotype is not valid for other plant forms. Therefore, each new investigated plant genotype requires the development of its own in vitro system. The effectiveness of the realization of the morphogen potential of various genotypes is determined by the concentration and ratio of phytohormones in the nutrient medium, without which it is impossible to reproduce plants in vitro (Kudoiarova et al., 2000; Vetchinkina & Mamayeva, 2005; Fomenko, & Maljush, 2010; Konvalyuk et al., 2010).
The research problem of this study is to examine the dependence of the morphogen development of explants of Shrub garden class roses, cultivated in vitro, on changes in the concentrations of cytokinins and auxins included in the composition of nutrient media.
Materials and Methods
Studies of the morphogen development of explants of the Shrub roses were carried out in the laboratory of microclonal reproduction of plants of the National Dendrological Park "Sofiyivka" NAS of Ukraine. When cultivating the plants in vitro, the method of microclonal reproduction was used, based on the induction of morphogen processes in explants under the influence of phytohormones (Kefeli, 1966; Kalinin et al., 1980; Vysotskii, 1986; Dixon & Gonzales, 1995; Pilunskaia & Bugara, 2001; Molkanova et al., 2002; Lavrentyeva, 2004; Rebrov & Gromova, 2008; Hrechanyk et al., 2011; Mitrofanova, 2011; Koldar, 2012; Orazbaeva et al., 2012; Kruglova & Seldimirova, 2013; Koldar et al., 2015; Nebykov et al., 2015). During the experiment, the Shrub class rose cultivars from the collection fund of the National Dendrological Park "Sofiyivka" NAS of Ukraine were used: ‘Gärtnerfreude’ (W. Kordes’ Söhne, 1991), ‘Lavender Dream’ (G. P. Ilsink, 1984), ‘Pomponella’ (W. Kordes’ Söhne, 2005), ‘Red Cascade’ (R. S. Moore, 1976), ‘Sommerabend’ (W. Kordes’ Söhne, 1995).
Based on the previous experience of in vitro propagation of roses of other garden classes, the propagation material was harvested in the phase of intensive development of shoots, when the latter was already in a semi-lignified state. In the natural and climatic conditions of the Right-Bank Forest-Steppe Zone of Ukraine, it was during this period that the maximum increase in the ability of the explants of roses to direct organogenesis was observed (Небиков et al., 2007; Koldar et al., 2009; Denysko, 2016).
The plant material was taken from 6-14-years-old paternal plants presented at the collection and exposition plots of the National Dendrological Park "Sofiyivka" NAS of Ukraine. For an introduction into in vitro culture, one-year shoots were used, from which microshoots 5-15 mm long were harvested and planted on nutrient media. The explants were cultivated on a universal essential nutrient medium according to the Murashige & Skoog (MS) prescription, which contains a balanced concentration of nutrients that promotes the growth in vitro of isolated tissues of many plants (Murashige & Skoog, 1962; Butenko, 1999; Kushnir & Sarnats'ka, 2005). The ability of explants to morphogenesis was studied upon exogenous addition of phytohormones to media at various concentrations: 6-benzylaminopurine (6-BAP), β-indolylbutyric acid (β-IBA), β-indoleacetic acid (β-IAA), 1-naphthylacetic acid (1-NAA). During the cultivation of the explants, the influence of the nutrient medium components on different morphogenesis stages was observed. The formula calculated the multiplication rate (m. r.) of microshoots of the studied rose cultivars:
M=a/bc,
where: M – multiplication rate,
a – number of shoots formed,
b – number of shoots planted,
c – number of passages (Romadanova et al., 2017).
To obtain sterile viable plant material, two-stage sterilization was carried out. Pretreatment was carried out using a neutral disinfectant BTC 885 (IPAX CLEANOGEL, USA; active substance – compounds of ammonium chloride salts in a concentration of 21.7%) with bactericidal and fungicidal properties, and the main treatment was carried out with the treatment of mercury dichloride (HgCl2) with the addition of the emulsifier Tween-80 (Scharlau Chemie, Spain) with an exposure of 1.0, 1.5 and 2.0 min. In each variant of the study, 30 microshoots were used. After decontamination, microshoots were planted on a hormone-free MS nutrient medium. Within 5–8 days, the sterilization efficiency was defined: the proportion of sterile and infected objects. The viability of the introduced explants was assessed after 10–14 days. The maximal proportion of sterile, viable explants was obtained after 1.5 min. Treatment of plant material with mercury dichloride (HgCl2): the maximum percentage of aseptic explants of the studied cultivars was 72.7–86.4%, viable ones – 64.8–78.8%, and the level of sterilization efficiency by cultivars were in the range of 59.8–74.7% (Table 1).
| Exposure, min. | Explant yield, % | Sterilization efficiency, % | |
|---|---|---|---|
| aseptic | viable | ||
| cv. 'Gärtnerfreude' | |||
| 1.0 | 73.3±0.14 | 68.4±0.14 | 60.4±0.22 |
| 1.5 | 79.7±0.16 | 73.8±0.11 | 72.3±0.14 |
| 2.0 | 86.6±0.21 | 52.2±0.09 | 28.8±0.19 |
| cv. 'Lavender Dream' | |||
| 1.0 | 70.3±0.23 | 77.8±0.13 | 59.8±0.18 |
| 1.5 | 86.4±0.18 | 72.6±0.12 | 73.6±0.09 |
| 2.0 | 84.4±0.21 | 47.4±0.16 | 54.2±0.11 |
| cv. 'Pomponella' | |||
| 1.0 | 79.8±0.17 | 71.0±0.15 | 65.3±0.22 |
| 1.5 | 83.6±0.19 | 78.8±0.16 | 74.7±0.16 |
| 2.0 | 64.7±0.21 | 51.7±0.18 | 52.7±0.12 |
| cv. 'Red Cascade' | |||
| 1.0 | 68.9±0.15 | 57.7±0.23 | 50.3±0.24 |
| 1.5 | 72.7±0.22 | 64.8±0.14 | 63.6±0.19 |
| 2.0 | 77.1±0.18 | 47.3±0.19 | 42.9±0.21 |
| cv. 'Sommerabend' | |||
| 1.0 | 77.2±0.14 | 61.4±0.18 | 57.3±0.17 |
| 1.5 | 78.1±0.12 | 68.9±0.22 | 59.8±0.11 |
| 2.0 | 77.5±0.13 | 56.8±0.26 | 33.9±0.15 |
Table 1. The efficiency of sterilization of microshoots of the Shrub rose class representatives
When selecting the optimal conditions for micropropagation and achieving the morphogen activity of plants, sterile viable explants of the studied cultivars were transferred to nutrient media supplemented with phytohormones of the auxin (β-IBA, β- IAA, 1-NAA) and cytokinin (6-BAP) groups. In the course of the experiment, the media of 13 variants were tested using the nutrient medium pH 5.7 (Table 2).
| The variant of nutrient medium | Phytohormone, mg/l | |||
|---|---|---|---|---|
| 6-BAP | β-IBA | β-IAA | 1-NAA | |
| I (control) | – | – | – | – |
| II | 0.5 | – | – | – |
| III | 0.5 | 0.01 | – | – |
| IV | 0.5 | – | 0.1 | – |
| V | 0.5 | – | – | 0.1 |
| VI | 1.0 | – | – | – |
| VII | 1.0 | 0.01 | – | – |
| VIII | 1.0 | – | 0.1 | – |
| IX | 1.0 | – | – | 0.1 |
| X | 2.0 | – | – | – |
| XI | 2.0 | 0.01 | – | – |
| XII | 2.0 | – | 0.1 | |
| XIII | 2.0 | – | – | 0.1 |
Table 2. Phytohormonal composition of modified nutrient media
Preparation of nutrient media, instruments, and materials, sterilization and cultivation of plant material were carried out using the methods and recommendations by Kalinin, Butenko, Kunakh (Kalinin et al., 1980; Kataeva & Butenko, 1983; Бутенко, 1999; Kunakh, 2005). The plants were cultivated at a temperature of 23±2 °C, the illumination intensity of 3000 lx, and a 16-hour photoperiod. Observation of the emergence and formation of morphological structures was carried out during the cultivation of the explants in vitro.
Results and Discussion
The main factors that determine the morphogen potential of explants are the plant genotype, stages of the cell cycle, cell metabolism, and endogenous growth regulators. The morphogen potential is also influenced by the organ from which the explant was obtained for further reproduction, the age of the donor plant, preparation and method of placement onto the medium. The cellular basis of morphogenesis is cytodifferentiation, and the most potent inducer of morphogenesis is a change in the ratio of cytokinins and auxins, which are components of nutrient media (Gamburg et al., 1978; Podvigina et al., 2001; Zelenina, 2005; Rugini & Pesce, 2006; Vlasenko et al., 2009; Bhojwani, S.S., & Dantu, 2013).
The possibility of rapid propagation of plants in vitro is provided by the ability of plant explants to regenerate (totipotency). Numerous studies have shown that the phenomenon of totipotency in plants is not always realized, since the potential capabilities of various cell types are not developed equally (Kalinin et al.,1980; Лаврентьєва, 2004; Mel'nychuk et al., 2003; Kunakh, 2005; Zhuravlev & Omel'ko, 2008).
The transfer of plant tissues to the culture in vitro means the termination of their existence as one of the structural elements of the whole organism, which they were part of earlier. Growth conditions and nutrition nature are undergoing significant changes. It should be noted that when introduced into the culture in vitro, explants separated from the maternal plant disturb the stability of the internal status, coordination of reactions aimed at maintaining dynamic equilibrium. In this case, a biological feature inherent in living organisms is of great importance – homeostasis – the tendency of the system to restore itself, to renovate the lost balance (Rebrov, & Gromova, 2008). Therefore, in explants separated from the mother plant, the coordination of cell and tissue interactions and the regulation of physiological and morphogen processes occur due to the content in nutrient media of appropriate concentrations of phytohormones that promote active regeneration and recovery of lost parts by the plant. Thus, when propagating arboreous plants in vitro culture, sometimes a large number of problems arise.
These studies aimed to modify nutrient media and observe changes in qualitative characteristics in explants of the Shrub roses, depending on the ratio of phytohormones in the nutrient media. During the experiment, when the MS medium was modified by adding various concentrations of phytohormones, differentiation of meristemic tissues took place, followed by the formation of conglomerates with shoot buds directly on the explant.
Using areas of shoots with axillary and apical buds as explants, morphogen structures that were formed according to the type of direct morphogenesis were obtained. As reported by Kushnir & Sarnatska, Kunakh, these structures were substantially genetically homogeneous and identical to the maternal form (Kunakh, 2005; Kushnir & Sarnats'ka, 2005).
For differentiation of meristemic tissues and induction of morphogenesis, viable explants were transferred to the MS nutrient medium with different phytohormone content. During 14–18 days of explantation, initiation of adventitious buds was observed in all variants of nutrient media (Figure 1), followed by the formation of shoots, which after 24–28 days reached a length of 10.9– 11.7 mm (Figure 2a).

Fig 1: The beginning of germination of adventitious buds in the cv. 'Lavender Dream' explant
With the further cultivation of the explants on nutrient media, the vegetative mass's active shoot formation and growth were observed. On the 37th–44th days of explants cultivation in all variants of the nutrient medium, further growth of shoots and increased size were observed. On the 40th–48th days of the cultivation, 3–8 microshoots were formed from each planted explant. They had green pigmentation, were without visual deformations, and reached a length of 10.8–20.4 mm (Figure 2b).
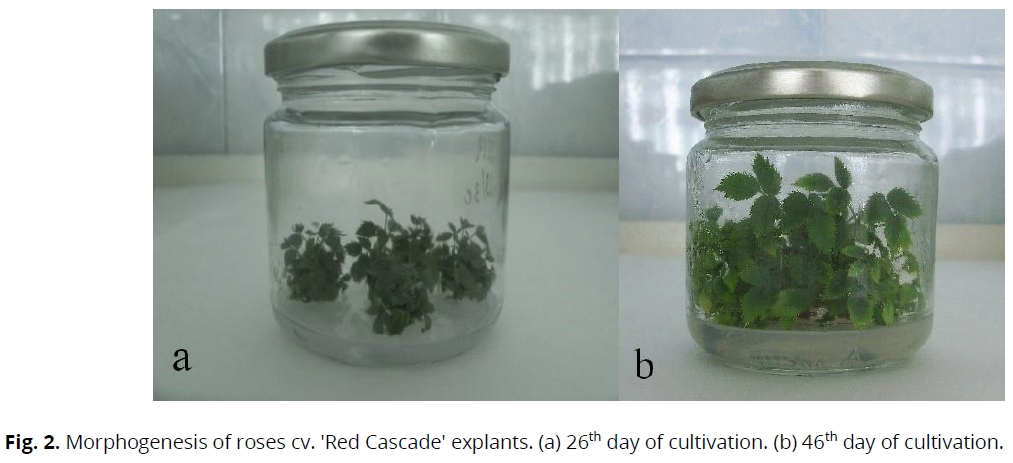
Fig 2: Morphogenesis of roses cv. 'Red Cascade' explants. (a) 26th day of cultivation. (b) 46th day of cultivation.
It was found that the multiplication rate depended on the content and concentrations of phytohormones in the nutrient media. Evaluation of the effectiveness of their effect was carried out after the second and subsequent passages, which made it possible to identify variants with a high multiplication rate (Table 3). At each passage, a tendency towards an increase in the m.r. was observed.
| The variant of nutrient medium | Number of shoots formed | Shoot length, cm | Multiplication rate |
|---|---|---|---|
| cv. ‘Gärtnerfreude’ | |||
| III | 3.62±0.18 | 1.90±0.07 | 3.66±0.15 |
| VII | 4.01±0.26 | 2.43±0.11 | 4.56±0.22 |
| XI | 5.39±0.12 | 3.47±0.08 | 5.42±0.12 |
| cv. 'Lavender Dream' | |||
| VII | 4.26±0.21 | 1.87±0.08 | 3.89±0.16 |
| IX | 4.67±0.12 | 2.09±0.09 | 4.98±0.23 |
| XI | 5.69±0.12 | 1.73±0.08 | 6.33±0.11 |
| cv. ‘Pomponella’ | |||
| IX | 4.12±0.19 | 1.83±0.07 | 3.77±0.18 |
| XI | 4.66±0.21 | 2.29±0.10 | 5.34±0.21 |
| XII | 2.92±0.14 | 1.86±0.08 | 3.72±0.13 |
| cv. 'Red Cascade' | |||
| VII | 7.70±0.19 | 1.43±0.12 | 8.0±0.33 |
| VIII | 8.40±0.16 | 1.74±0.08 | 9.2±0.21 |
| XI | 12.04±0.09 | 2.10±0.16 | 12.2±0.33 |
| cv. 'Sommerabend' | |||
| IX | 5.23±0.25 | 2.01±0.08 | 5.26±0.25 |
| XI | 6.61±0.31 | 2.19±0.10 | 7.24±0.35 |
| XII | 3.22±0.12 | 1.94±0.09 | 3.12±0.13 |
Table 3. Multiplication rate of the Shrub roses depending on the content of phytohormones in nutrient media (average for six passages)
During the study of the morphogen ability of the studied objects using explants of various taxa and ratios in nutrient media of growth regulators, differences in the formation of adventive buds, their number and intensity of growth, and the size of leaves were observed. Explants of different genotypes formed vegetative organs that differed both in appearance and morphogen ability. The frequency of the emergence of new organs under in vitro conditions varied depending on the type of explants, plant genotype, and nutrient medium composition. These results indicate that only a specific concentration of phytohormones can be effective for a particular genotype, or such a genotype is sensitive and reacts positively only to a specific ratio of the phytohormones.
The highest indices of morphogen potential were found in explants of the studied cultivars developed on a nutrient medium containing 6-BAP – 2.0 mg/l and β-IBA – 0.01 mg/l (variant XI), which promoted active passing of the processes of morphogenesis in explants. Thus, on the 40th–46th days of the explants cultivation, a high multiplication rate (12.04±0.33) was found in the cv. 'Red Cascade' (Figure 2b). The cvs. 'Sommerabend', 'Lavender Dream', 'Gärtnerfreude', 'Pomponella' also exhibited morphogen activity; however, the m. r. was slightly lower and averaged, respectively 7.24±0.35; 6.33±0.11; 5.42±0.12 and 5.34±0.21.
When added to nutrient media 1.0 mg/l 6-BAP and 0.01 mg/l β-IBA (variant VII), the morphological activity of explants decreased, which led to a slight diminution in the multiplication rate indices. It was the highest in the cv. 'Red Cascade' – 8±0.33, but in the cv. ‘Gärtnerfreude’ and cv. 'Lavender Dream' it was 4.56±0.22 and 3.89±0.16, respectively. The cv. 'Red Cascade' had a high morphogen potential on a medium containing 6-BAP 1.0 mg/l and 0.1 mg/l β-IBA (variant VIII), where the multiplication rate was 9.2±0.21. The cvs. 'Gärtnerfreude', 'Lavender Dream', 'Pomponella' under the influence of different concentrations of phytohormones in the variants III, IX, and XII (Table 1), had slightly lower indices of the morphogen potential of plants with m. r. within 3.66±0.15 – 6.33±0.11. The analysis of the obtained results shows that the representatives of the studied garden class of roses belong to taxa with high morphogen ability. The efficiency of the morphogenesis processes in explants can be optimized by cultivating them on nutrient media specific for phytohormonal composition. The medium with the content of 6- BAP 2.0 mg/l and β-IMA – 0.01 mg/l (variant XI) was the best of the tested ones. Among the studied cultivars of the Shrub roses, the most active passaging of the morphogenesis processes on this medium occurred in the cvs. 'Red Cascade' and 'Sommerabend'.
Conclusion
Thus, differentiation of meristemic tissues with subsequent induction of morphogenesis in rose cultivars of the Shrub garden class depended on the concentration of phytohormones in the nutrient medium. The use of shoots with axillary and apical buds contributed to the formation of morphogen structures formed directly on the explants by the type of direct morphogenesis. Among the tested media, the most favorable for the induction of morphogenesis and the achievement of high multiplication rate in vitro was the variant, supplemented with 2.0 mg/l 6-benzylaminopurine and 0.01 mg/l β-indolylbutyric acid and the variant containing 1.0 mg/l 6-benzylaminopurine and 0.1 mg/l β-indolylbutyric acids. It was found out that the explants of cv. 'Red Cascade' and 'Sommerabend' showed a high morphogen ability (m. r. 12.2±0.33 and 9.2±0.21). The cvs. 'Gärtnerfreude', 'Lavender Dream', 'Pomponella', when exposed to different concentrations of phytohormones, had slightly lower rates adventitious shoot formation, and the m. r. was in the range of 3.66±0.15 – 6.33±0.11. We proved the prospects for successful reproduction of these plants in vitro, and the short reproduction cycle allows for a short time to obtain plants with the maintaining of the necessary maternal characteristics and makes it possible to produce mass planting material for their wide use in landscaping settlements of the Right-Bank Forest-Steppe Zone of Ukraine.
References
Beals, P. (1997). Classic Roses: An Illustrated Encyclopedia and Grower's Manual of Old Roses, Shrub Roses and Climbers. London: Harvill Press.
Bhojwani, S.S., & Dantu, P.K. (2013). Plant tissue culture: An introductory text. New Delhi: Springer.
Bumbeeva, L.I. (2010). Rozy [Roses]. Moscow: Kladez'-Buks (in Russian).
Butenko, R.G. (1999). Biologiia kletok vysshikh rastenii in vitro i biotekhnologiia na ikh osnove [Cell biology of higher plants in vitro and biotechnology based on them]. Moscow: FBK-PRESS (in Russian).
Cairns, T. (2003). Horticultural Classification Schemes. In.: Roberts, A.V., Debener, T, & Gudin, S. (Eds.) Encyclopedia of Rose Science (Vol. 1, pp. 117–124). Elsevier.
Denysko, I.L. (2016). Troiandy patio. Bioloho-ekolohichni osoblyvosti, introduktsiia, perspektyvy vykorystannia u Pravoberezhnomu Lisostepu Ukrainy [Patio roses. Biological and ecological characteristics, introduction, prospects to use in the Right-Banc Forest-Steppe Zone of Ukraine]. Kyiv: Palyvoda A.V. (in Ukrainian).
Dixon, R.A., & Gonzales, R.A. (Eds.). (1995). Plant Cell Culture: A Practical Approach (2nd ed.). Oxford University Press, U.S.A.
Fomenko, T.I., & Maljush, M.K. (2010). Morphogenesis peculiarity and plant regeneration in vitro culture of narrow-leafed lupin (Lupinus angustifolius L.). Physiology and biochemistry of cultivated plants, 42(4), 306–314 (in Russian).
Gamburg, K.Z., Leonova, L.A., & Rekoslavskaia, N.Iu. (1978). Metabolizm auksinov v roste kul'tur rastitel'nykh kletok [Metabolism of auxins in the growth of plant cell cultures]. In Kul'tura kletok rastenii [Plant cell culture] (pp. 47–52). Kyiv: Naukova dumka (in Russian).
Hrechanyk, R.М., Guz, M.M., & Oleksiychenko, N.О. (2011). Mulberry white (Morus alba Linn.) peculiarities introduction in culture in vitro. Scientific Bulletin of UNFU, 21(17), 9–21 (in Ukrainian).
Jacobi, K. (2002). Roses (A. Shackleton, Transl.). Bicester: Aura Books. (Original work published 1992).
Kalinin, F.L., Sarnatskaia, V.V., & Polishchuk V.E. (1980). Metody kul'tury tkanei v fiziologii i biokhimii rastenii [Tissue culture methods in plant physiology and biochemistry]. Kyiv: Naukova dumka (in Russian).
Kataeva, R.V., & Butenko, R.N. (1983). Klonal'noe mikrorazmnozhenie rastenii [Clonal micropropagation of plants]. Moscow: Nauka (in Russian). Kefeli, V.Iu. (1966). Novye dannye ob endogennoi reguliatsii rosta rastenii [New data on endogenous regulation of plant growth]. Agricultural
Chemistry, 7, 127–139 (in Russian).
Koldar, L.A. (2012). The role of phytohormones in the determination of Cerasus serratula Lindl. explants cultivated in vitro. News Biosphere Reserve "Askania Nova", 14, 152–155 (in Ukrainian).
Koldar, L.A., Nebykov, M.V., & Andrienko, O.D. (2015). Organogenesis induction from explants Amelanchier ovalis Medik. in vitro. Native and alien plant sciences, 11, 100–105 (in Ukrainian).
Koldar, L.A., Nebykov, M.V., & Denysko, I.L. (2009). Experimental organogenesis of patio roses in the conditions of in vitro culture. Native and alien plant sciences, 5, 114–118 (in Ukrainian).
Konvalyuk, I.I., Kravets, N.B., Drobyk, N.M., Mel’nyk, V.M., & Kunakh, V.A. (2005). Direct organogenesis in vitro of Gentiana lutea L. Biotechnologia Acta, 3(5), 66–73 (in Ukrainian).
Koval'chuk, I.Iu., Mukhitdinova, Z.R., Turdiev, T.T., Uspanova, G.K., & Chukanova, N.I. (2008, August). Mikroklonal'noe razmnozhenie maliny, kak metod sokhraneniia bioraznoobraziia rastenii v Kazakhstane [Micropropagation of raspberries as a method of preserving plant biodiversity in Kazakhstan]. In Biotekhnologiia kak instrument sokhraneniia bioraznoobraziia rastitel'nogo mira [Biotechnology as an instrument for vegetable world biodiversity conservation]. 2nd All-Russian Scientific and Practical Conference (Russia), Volgograd (p. 67) (in Russian).
Kruglova, N.N., & Seldimirova, O.A. (2013). The pathways of morphogenesis in vitro of wheat androclynic callus cells. Plant physiology and genetics, 45(5), 382–389 (in Russian).
Kudoiarova, G.R., Teplova, I.R., Dokicheva, R.A., Iusmanov I.Iu., & Veselov S.Iu. (2000, October). Vliianie benzil–6–aminopurina na rost i soderzhanie auksinov v prorostkakh pshenitsy i kukuruzy [Effect of benzyl-6-aminopurine on the growth and content of auxins in wheat and maize seedlings]. In Immunoanaliz reguliatorov rosta v reshenii problem fiziologii rastenii, rastenievodstva i biotekhnologii
[Immunoassay analysis of growth regulators in solving problems of plant physiology, crop production and biotechnology]. 3d Conference (Russia), Ufa (p. 224).
Kunakh, V.A. (2005). Biotechnology of medicinal plants. Genetic, physiological and biochemical basis. Kyiv: Logos (in Ukrainian).
Kushnir, H.P., & Sarnats'ka V.V. (2005). Mikroklonal'ne rozmnozhennia roslyn. Teoriia i praktyka [Microclonal plant propagation. Theory and practice]. Kyiv: Naukova dumka (in Ukrainian).
Lavrentyeva, A.M. (2004). Application of biotechnologycal methods in propagation of ornamental plants introduced into Ukraine. Visnyk of L'viv University. Biological Series, 36, 137–145 (in Ukrainian).
Matskevych, V.V., Rohovs'kyj, S.V., Vlasenko, M.Yu., & Cherniak, V.M. (2010). Osnovy biotekhnolohii roslyn: navchal'nyj posibnyk [Plant biotechnology fundamentals: A study guide]. Bila Tserkva: BNAU (in Ukrainian).
Mattock, J. (1996). Gardener's Guide to Growing Roses. London: Reader's Digest.
Mel'nychuk, M.D., Novak, T.V., & Kunakh V.A. (2003). Biotekhnolohiia Roslyn [Plant biotechnology]. Kyiv: Polihraf Konsaltynh (in Ukrainian). Mitrofanova, Iu.V. (2011). Somaticheskii embriogenez i organogenez kak osnova biotekhnologii polucheniia i sokhraneniia mnogoletnikh
sadovykh kul'tur [Somatic embryogenesis and organogenesis as the basis of biotechnology for obtaining and preserving perennial horticultural crops]. Kyiv: Ahrarna nauka (in Russian).
Molkanova, O.I., Churikova, O.A., Konovalova, L.N., & Okuneva, I.B. (2002). Clonal micropropagation of the introduced varieties of Syringa vulgaris L. Herald of Moscow University. Series 16. Biology, 4, 8–14 (in Russian).
Moroz, O.K., & Denysko, I.L. (2010, October). Ekolohichni aspekty vykorystannia gruntopokryvnykh troiand v urboseredovyschi [Ecological aspects of Groundcover roses use in urban environment]. In Promyslova botanika: stan ta perspektyvy rozvytku [Industrial botany: state and development prospects]. 6th International Scientific Conference (Ukraine), Donetsk (pp. 313–315). Donetsk Botanical Garden NAS of Ukraine (in Ukrainian).
Moroz, O.K., & Denysko, I.L. (2014, June). Vykorystannia gruntopokryvnykh troiand u ozelenenni avtomobil'nykh dorih [Arrangement of highways with groundcover roses]. In Aktual'ni problemy ozelenennia naselenykh mists': osvita, nauka, vyrobnytstvo, mystetstvo formuvannia landshaftu [Problems of landscaping of populated areas: education, science, production, art of landscape formation]. 2nd International Scientific and Practical Conference (Ukraine), Bila Tserkva (pp. 70–73) (in Ukrainian).
Moroz, O.K., Denysko, I.L., & Bank, V.S. (2012). Collection of Shrub roses in the National Dendrological Park "Sofiyivka" NAS of Ukraine. News Biosphere Reserve "Askania Nova", 14, 181–185 (in Ukrainian).
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant., 15(13), 473– 497.
Nebykov, M.V., Koldar, L.A., & Derev'ianko, N.V. (2015, September). Rozmnozhennia Prunus laurocerasus in vitro [Reproduction of Prunus laurocerasus in vitro]. In Introduktsiya roslyn, zberezhennya ta zbahachennya bioriznomanittya v botanichnykh sadakh ta dendroparkakh [Plant introduction, conservation and enrichment of biodiversity in botanical gardens and arboreta]. International Scientific Conference (Ukraine), Kyiv (pp. 177–178). Fitosotsiotsentr (in Ukrainian).
Nebykov, M.V., Koldar, L.A., & Moroz, O.K. (2007). Rosa chinensis Jacq. minima (Sims) in culture in vitro. The Scientific Issues of Ternopil Volodymyr Hnatiuk National Pedagogical University. Series: biology, 3(33), 51–55 (in Ukrainian).
Orazbaeva, G.K., Khasanov, V.T., Iskhakov, A.R., & Shvidchenko, V.K. (2012). Klonal'noe razmnozhenie rastenii chernoi smorodiny (Ribes nigrum L.) in vitro [Clonal reproduction of black currant plants (Ribes nigrum L.) in vitro]. Science Bulletin of S. Seifullin Kazakh Agrotechnical University, 1(72), 115–124 (in Russian).
Pilunskaia, O.A., & Bugara, A.M. (2001). Micropropagation of essential oil rose in vitro. Bulletin of the State Nikitsky Botanical Gardens, 83, 84- 86 (in Russian).
Podvigina, O.A., Znamenskaia, V.V., & Frolova, V.V. (2001). Induktsiia rizogeneza u sakharnoi svekly v kul'ture in vitro [Induction of rhizogenesis in sugar beet in culture in vitro]. In Reguliatory rosta i razvitiia rastenii v biotekhnologii [Plant growth and development regulators in biotechnology]. 6th International Scientific Conference (Russia), Moscow (p. 160). Moscow Timiryazev Agricultural Academy (in Russian).
Rebrov, V.G., & Gromova, O.A. (2008). Vitaminy, makro- i mikroelementy [Vitamins, macro- and microelements]. Moscow: GEOTAR-Media (in Russian).
Romadanova, N.V., Seraj, N.A., Nurmanov, М.М., & Karasholakova, L.N. (2017). Introduction of wild Malus sieversii apple into in vitro culture. Research, results, 3(75), 103–110 (in Russian).
Rugini, E.A., & Pesce, P.G. (2006). Genetic improvement of olive. Pomologia Croatica, 12(1), 43–74. Valikhanova, G.Zh. (1996). Biotekhnologiia rastenii [Plant biotechnology]. Almaty: Konzhyk (in Russian).
Vetchinkina, E.M., & Mamayeva, N.A. (2005). Some aspects of the use of embryoculture of the genus Iris L. Bulletin Taras Shevchenko National University of Kyiv. Series Introduction and Conservation of Plant Diversity, 9, 15–16 (in Russian).
Vlasenko, M.Yu., Matskevych, V.V., Dul'niev, P.H., & Kozak, A.L. (2009, June). Determinatsiia ontohenezu roslyn kartopli v umovakh in vitro syntetychnymy fitohormonamy klasu tsytokininiv [Ontogenesis determination of potato plants in vitro by synthetic phytohormones of cytokinin class]. In Innovatsijni ahrotekhnolohii v umovakh hlobal'noho poteplinnia [Innovative agricultural technologies in the context of global warming] International Scientific and Practical Conference (Ukraine), Melitopol, Kyrylivka, 1, 24–25. Tavria State Agrotechnological University (in Ukrainian).
Vysotskii, V.A. (1986). Klonal'noe mikrorazmnozhenie rastenii. Kul'tura kletok rastenii i biotekhnologiia [Clonal micropropagation of plants. Plant cell culture and biotechnology]. Moscow: Nauka (in Russian).
Zelenina, H.A. (2005). Effect of nutrient medium components on micropropagation efficiency of Arnica foliosa Nutt. in vitro. The Bulletin of Kharkiv National Agrarian University. Series biology, 2(7), 89–93 (in Ukrainian).
Zhuravlev, Iu.N., & Omel'ko, A.M. (2008). Morfogenez u rastenii in vitro [Plant morphogenesis in vitro]. Plant Physiology, 55(5), 643–664 (in Russian).
Author Info
S. Kosenko1, L. A. Koldar1, I. L. Denysko1, O. A. Balabak1, M. V. Nebykov1, A. F. Balabak2 and A. V. Balabak2*2Uman National University of Horticulture, 1 Instytuts'ka St, Uman, Ukraine
Citation: Kosenko, I.S., Koldar, L.A., Denysko, I.L., Balabak, O.A., Nebykov, M.V., Balabak, A.F., Balabak, A.V. (2021). Morphogen development of in vitro cultivated Shrub roses. Ukrainian Journal of Ecology, 11 (2), 229-235.
Received: 11-Mar-2021 Accepted: 18-Apr-2021 Published: 30-Apr-2021, DOI: 10.15421/2021_104
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
