Research Article - (2023) Volume 13, Issue 1
Phenology and spatio-temporal distribution of Ardeidae in Lac Tonga (North-eastern Algeria)
L. Ouarti1*, N. Nouri1, A. Lazli1, K. Missaoui2 and M. Houhamdi3Abstract
In this study, a 2600-ha Ramsar site, Lac Tonga, in northeastern Algeria, was fortnightly surveillance, for seven months, from December 2021 to June 2022. The aim was to monitor and determine the spatio-temporal distribution of Ardeidae. A total of seven species were inventoried, including the Western Cattle egret Ardea ibis (1701 individuals) and the Little egret Egretta garzetta (265 individuals). The other species were the Squacco heron Ardeola ralloides, Grey heron Ardea cinerea, Purple heron Ardea purpurea, Black-crowned Night heron Nycticorax nycticorax and Great egret Ardea alba, with 69, 56, 54, 51 and 35 individuals respectively. During the study period, maximum abundance was observed during May (A=582 individuals), and species richness generally varied between 3 and 5 species. The highest diversity and equitability indices values were noted during June (H'=1.4 bits and E=0.88), indicating the presence of a balanced population. The monitoring of Ardeidae’ spatial distribution shows that they exploit mainly the western and northeastern parts of the lake with an important occupation during the study period.
Keywords
Wetland, Lac Tonga, Ardeidae, spatio-temporal distribution, diversity.
Introduction
The Mediterranean region is considered one of the hotspots of biodiversity. It is known for the diversity of its flora and fauna, including aquatic birds, which are one of the components of its landscape (Saifouni, 2009). Knowledge of the breeding sites of these waterbirds, their distribution through the various habitats, during or outside the nesting season, and their behavior, allows the detection of any decline or imbalance of these natural environments (Arendt, 1988; Boisteau and Marion, 2006). Algeria shelters a great diversity of wetlands which are important stations for migratory, breeding and wintering birds (Samraoui, et al., 2006; Mohamed Ali, et al., 2008). The number of Ardeidae has increased significantly in several countries of the Mediterranean region (Marion and Duhautois, 1986; Fasola, et al., 2011). The northeastern part of Algeria contains the largest number of wetlands (72) (Gherib, et al., 2021). Many of them are listed in Ramsar, and they play a very important role in the populations of Ardeidae. It is due to the diversity and heterogeneity of the vegetation that also provides shelter from the weather and predators and increases the density of prey such as reptiles (snakes, lizards), amphibians (frogs), or fish (Chettibi, et al., 2019). The vast majority of Heron colonies in the Numidia region (northeastern Algeria) are often observed in the wetland complex of El Kala, such as Lac Tonga (Samraoui-Chenafi, 2009).
Very few studies have been devoted to studying this aquatic avifauna’s habitat and ecological niche during wintering. Existing works are mainly based on the monitoring of wintering behavior (De Belair, 2005; Houhamdi and Samraoui, 2008; Houhamdi and Samraoui, 2001; Boukrouma, et al., 2011). In Algeria, ten species of herons are inventoried (Isenmann and Moali, 2000; Samraoui and Samraoui, 2008): Egretta gazetta, Ardea purpurea, Ardea ibis, Ardea alba, Ardeola ralloides, Ixobrychus minutus, Nycticorax nycticorax, Botaurus stellaris, Ardea cinerea, Egretta gularis, which is recently observed in the Mekhada marsh (Ramsar site) and southern Algeria (Ghardaïa and El-Ménéa region) (Telailia, et al., 2018; Chedad, et al., 2021 a; Gherib, et al., 2021).
The objective of this work is two fold: (i) monitoring the dynamics of the Ardeidae population of Lac Tonga through an inventory, a count of the numbers and the determination of ecological indices, allowing to characterize this population; (ii) studying the different modalities of spatio-temporal distribution of the species recorded in the water body and more particularly in the western part because it is the zone that shelters the most these species. This survey can be used as a database for future studies and wetland management (Fig. 1).
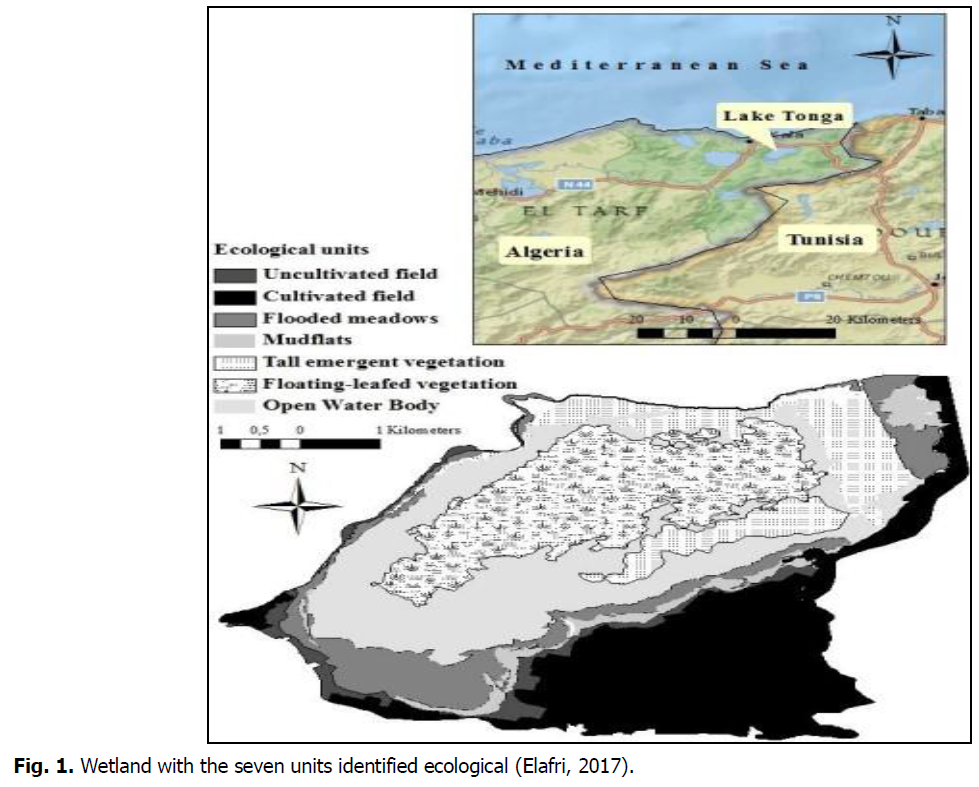
Fig 1: Wetland with the seven units identified ecological (Elafri, 2017).
Site description
Our study was conducted at Lac Tonga (36°53’N, 08°31’E, altitude 2 m) (Fig. 2), which was designated a Ramsar site in 1983 (Sarri, 2017) and an integral reserve of El Kala National Park (Loucif, 2020). Located in the extreme northeast of Algeria, it is a freshwater marsh with an area of 2600 ha and an average depth of 120 cm (Chettibi, 2020). It is fed by two tributaries: Oued El Hout from the southeast and Oued El Eurg from the east (Bakaria et al., 2018). On the northern part of Lac Tonga is the artificial channel of the Messida (Lazli et al., 2011a,b; Chettibi, 2020). The study site’s prevailing climate is sub-humid (Elafri, 2017; Loucif, 2020; Gherib et al., 2021). Lac Tonga is characterized by a great heterogeneity of vegetation formations, constituting nesting sites for many aquatic birds while providing them with rich and varied trophic resources (Kadid, 1989). Examples are the willow grove, which extends from the northeast and southeast shore of the lake towards the center, the large Reed Forest, and the large Water Lily carpet, which is dominated in the center, especially during the breeding season (Menasria and Lazli, 2017; Saifouni et al., 2020; Nouri et al., 2022).
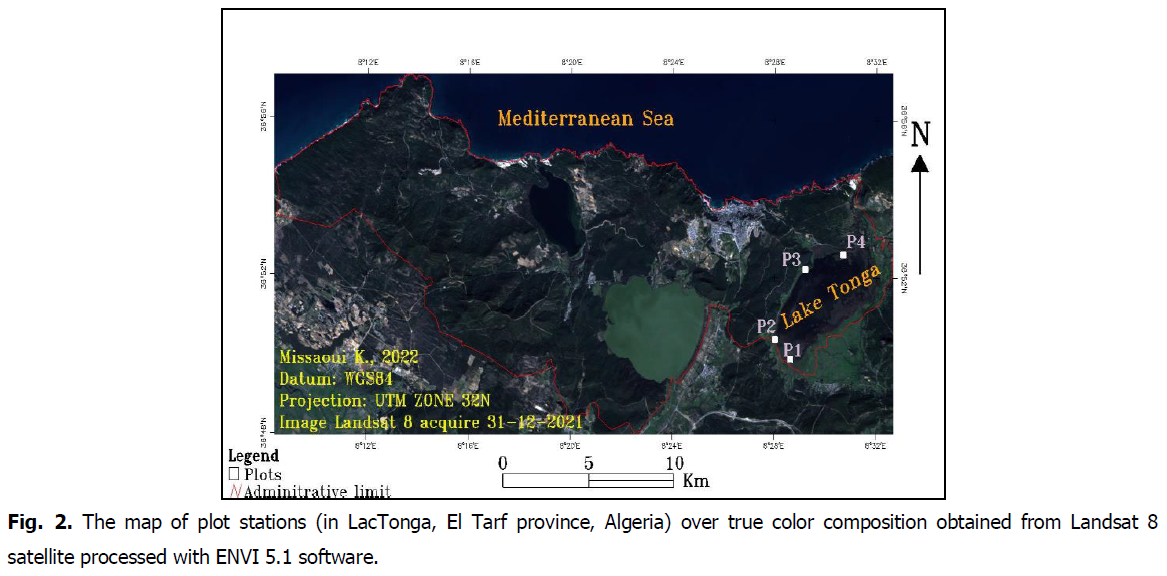
Fig 2: The map of plot stations (in LacTonga, El Tarf province, Algeria) over true color composition obtained from Landsat 8 satellite processed with ENVI 5.1 software.
Materials and Methods
This study was conducted over seven months between December 2021 and June 2022. Plot investigations were conducted with a telescope (Konus-Spot 20 x 60) and a pair of binoculars (KERN, 8 x 30). Monitoring was conducted from four observation points, which covered the entire wetland and met the criteria of good visibility, accessibility and location of the Ardeidae. A Garmen GPS was used to locate the observation points. Counts were performed fortnightly using the methods described in the literature (Blondel, 1975; Legendre and Legendre, 1979). The count was individual when the group of birds was less than 200 m away and a quantitative estimate when the groups were further away (Lamotte et Bourliére, 1969; Blondel, 1975). A bird identification guide (Heinzel Guide of Europe Birds) was used to facilitate the identification of Ardeidae species. We avoided rainy days, minimized sudden movements and operated in silence. One observer conducted the count early in the morning for about 7 h.
At Lac Tonga, the spatial position of Ardeidae species and their colonies were mapped using GPS. These data allowed us to identify species’ habitat selection in contrast to others observed throughout the water body.
(i) total abundance N: the total number of individuals of all species (Dajoz, 1971). (ii) total species richness S: the total number of species surveyed. The number of bird species observed at a specific date allows the identification and characterization of periods when the number of birds is greatest (Blondel, 1969; Legendre and Legendre, 1979; Lindström and Piersma, 1993). The average specific richness Sm is useful in studying the structure of stands. It is calculated by the average number of species present in a sample (Ramade, 1984), Sm=total number of species surveyed at each survey number of surveys conducted. (iii) Frequency number: constancy is the ratio of the number of surveys containing the species studied to the total number of surveys, expressed as a percentage. It lets us know how the species is distributed: Omnipresent, Constant, Common, Accidental, very accidental species and: FN=(ni/N) x 100 (Dajoz, 1971; Bachelier, 1978). (iv) Shannon-Weaver diversity index: H′=[ΣPi ln Pi], where Pi=is the proportion of each species in the sample (Daget, 1979). H’ value varies from 0 when the community is composed of only one species to 4.5 or 5 bits for the most diverse communities; the lowest values, less than 1.5 bits, are associated with communities dominated by 01 or a few species (Faurie, et al., 2003 in Gherib et al., 2021). (v) Equitability index: E=H’/Hmax where Hmax=ln S. The equity index is between 0 and 1 (Pielou 1969). When E is close to 1, diversity and abundance are close to balance (Legendre and Legendre, 1979).
Minitab 21.3.0 statistical software and Excel version 2211 were used to calculate these indices.
Regarding cartography, we used data from the Landsat 8 sensor. The imaging software used is ENVI 5.1. To get the true color composition we used red, blue, and green tape. The method consists in downloading the bands from the Landsat sensor, we cut the study area well in the town of El Tarf, by the polygons of the limits of our area of investigation. We have materialized the observation stations. Second map same procedure, but we have materialized the distribution of species of the Ardeidae family in the study area.
Results
During the study period in Lac Tonga, twenty-six species of waterbirds were recorded on this water body, including seven species of Ardeidae: Little egret, Night heron, Squacco heron, Great egret, Grey heron, Western Cattle egret and Purple heron (Table 1).
| Family | Common Name | Scientific Name | Conservation status | |
|---|---|---|---|---|
| Ardeidae | Little egret | Egretta gazetta | AEWA/A. L | |
| Grey heron | Ardea cinerea | AEWA | ||
| Western Cattle egret | Ardea ibis | AEWA | ||
| Purple heron | Ardea purpurea | AEWA/A. L | ||
| Great egret | Ardea alba | A. L | ||
| Night heron | Nycticorax nycticorax | AEWA/A. L | ||
| Squacco heron | Ardeola ralloides | AEWA/A. L | ||
| Podicipedidae | Little grebe | Tachybaptus ruficollis | AEWA | |
| Great Crested grebe | Podiceps cristatus | AEWA | ||
| Rallidae | Common moorhen | Gallinula chloropus | AEWA | |
| Common coot | Fulica atra | AEWA | ||
| Purple swamphen | Porphyrio porphyrio | A. L | ||
| Charadriidae | Northern lapwing | Vanellus vanellus | UICN (Vul)/AEWA | |
| Recurvirostridae | Black-winged stilt |
Himantopus himantopus | AEWA/A. L | |
| Phalacrocoracidae | Great cormorant |
Phalacrocorax carbo | AEWA/A. L | |
| Laridae | Whiskered tern |
Chlidonias hybrida | AEWA/A. L | |
| Black-Headed gull |
Larus ridibundus | – | ||
| Yellow-Legged gull |
Larus michahellis | – | ||
| Anatidae | Common teal | Anas crecca | AEWA | |
| Gadwall | Mareca strepera | AEWA | ||
| Eurasian wigeon | Mareca penelope | AEWA | ||
| Northern shoveler |
Spatula clypeata | AEWA | ||
| Mallard | Anas platyrhynchos | AEWA | ||
| White-Headed duck |
Oxyura leucocephala | UICN (End.)/AEWA/A. L | ||
| Common pochard | Aythya ferina | UICN (Vul.)/AEWA | ||
| Marbled duck | Marmaronetta angustirostris | UICN (Vul.)/AEWA/A. L |
Table 1. The list of waterbirds recorded at Lac Tonga (northeastern Algeria). Conservation status (A.L, Algerian legislation), The Agreement on the Conservation of African-Eurasian Migratory Waterbirds (AEWA), International Union for Conservation of Nature and Natural (endangered) (UICN (End.)), International Union for Conservation of Nature and Natural (Vulnerable) (UICN (Vul.)).
Most species of the Ardeidae inventoried were observed during the wintering season. Species countered showed different phenological statuses: winterers, sedentary breeders, summer breeders and sedentary non-breeders. Summer breeders were the most dominant, with about 42.86%, followed by sedentary breeders, with about 28.56%. Winterers and sedentary non-breeders were the least numerous at the site, accounting for about 14.29% of the observed Ardeidae (Fig. 3).
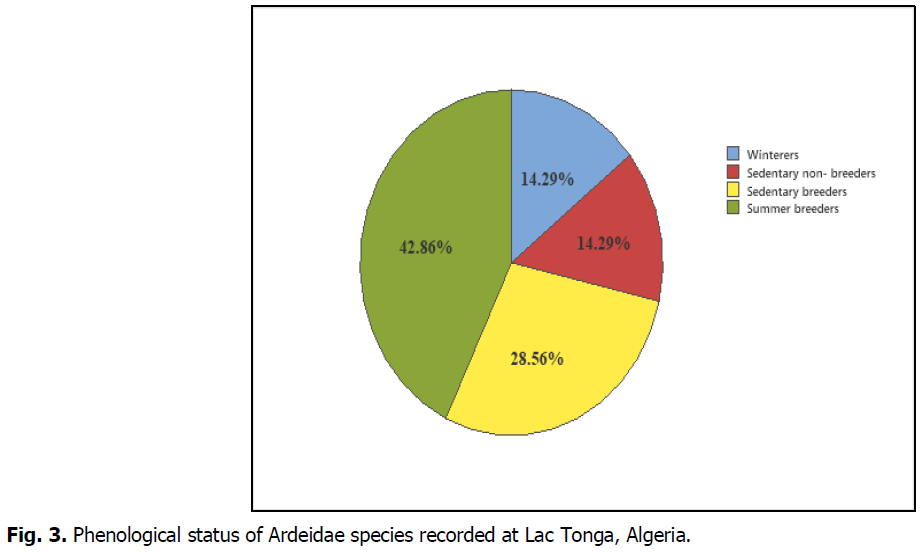
Fig 3: Phenological status of Ardeidae species recorded at Lac Tonga, Algeria.
Based on the research conducted in recent years and our observations during the study period, we determined the phenological status of the Ardeidae family at Lac Tonga. It can be noted that the Western Cattle egret is a sedentary breeder species in this water body (Table 2), present year-round. From December to early February, numbers were relatively low but more abundant in May, with a maximum of 494 individuals (Fig. 4).
| Species | Moy ± sd | Phenological status |
|---|---|---|
| Egretta garzetta | 18.286 ± 18.4577 | SB |
| Ardea cinerea | 4 ± 4.11377 | SNB |
| Ardea ibis | 121.5 ± 131.180 | SB |
| Ardea purpurea | 3.85714 ± 7.19890 | SmB |
| Ardea alba | 2.5 ± 3.27579 | W |
| Nycticorax nycticorax | 3.64286 ± 6.61708 | SmB |
| Ardeola ralloides | 4.92857 ± 6.48625 | SmB |
Table 2. Phenological status of Ardeidae recorded at Lac Tonga. W: Winterers, SB: Sedentary Breeder, SmB: Summers Breeder, SNB: Sedentary Non-Breeder.
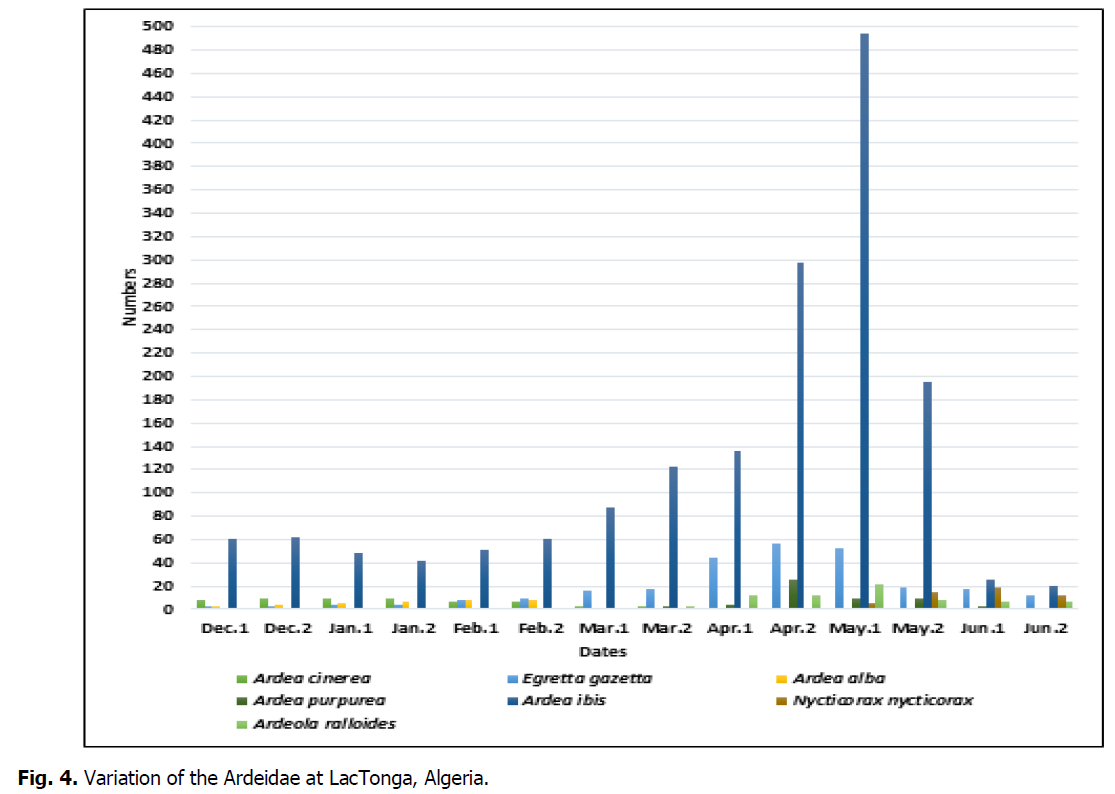
Fig 4: Variation of the Ardeidae at LacTonga, Algeria.
During the study period, Squacco heron numbers ranged from 3 to 21 individuals, with the largest numbers recorded from March to June. From December to February, the species was absent at Lac Tonga, which confirms its summer breeding status (Table 2). The Purple heron was only recorded from April to June, with a maximum of 26 individuals recorded in late April (Fig. 4). This species is a summer breeder in Lac Tonga (Table 2). In Lac Tonga, the presence of the Grey heron was recorded from December to April, with a maximum of 10 individuals (Fig. 4) noted during late December and January. This species is sedentary, not a breeder (Table 2).
The Great egret was observed from December to March, with numbers varying between 4 and 8 individuals (Fig. 4). The maximum number of individuals was recorded during February, suggesting that it is a wintering species (Table 2). The population of the Little egret is sedentary and breeds at this site (Table 2). The presence of the Night heron at Lac Tonga was reported during the nesting period (May-June), with a peak of 19 individuals recorded at the beginning of June (Fig. 4). This species, a summer breeder (Table 2), nests in this site with other Ardeidae (Fig. 5).

Fig 5: (a): Ardea ibis; (b): Ardea purpurea; (c): Egretta garzetta; (d): Ardeola ralloides; (e): Nycticorax nycticorax; (f): Ardea cinerea.
Monitoring of Ardeidae abundance at Lac Tonga showed values fluctuating from fortnight to fortnight. The maximum number of individuals for the wintering and breeding season was recorded in the first half of May, with 582 individuals, and the lowest number was recorded in the second half of June (Fig. 6).
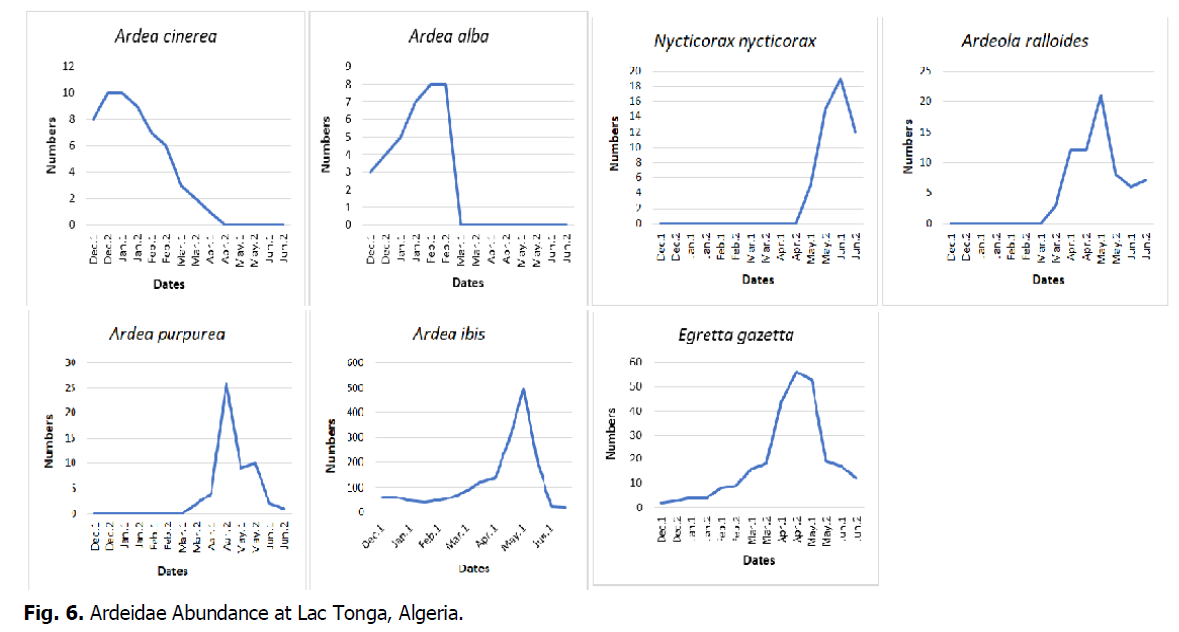
Fig 6: Ardeidae Abundance at Lac Tonga, Algeria.
In general, the species richness during the wintering season, to some extent, was stable at S=4. It increased to S=5 during the breeding season. The highest bi-monthly species richness was recorded in the second half of March and the first half of April (the end of the wintering season). In May and June, 5 species were recorded, suggesting the return of birds to their breeding grounds and the beginning of the breeding season for sedentary or summer species. The average richness varied between 13 in June and 145.5 in May. The highest values were noted during April and May.
According to the frequencies of occurrence, Bigot and Bodot (1973) classified the species into:
• Very accidental species: an occurrence of less than 10%.
• Accidental species occurrence: varies between 10 and 24%.
• Common species: are present in 25-49%.
• Constant species: are present in 50%.
• Omnipresent species (Omn): for more than 75% of species.
Based on this classification, we determined the frequency of each species inventoried (Table. 3).
| Species | Min | Max | Frequency of occurrence (C%) |
|---|---|---|---|
| Egretta garzetta | 02 | 56 | 100% (Omnipresent) |
| Ardea cinerea | 01 | 10 | 64.28% (Constant) |
| Ardea ibis | 40 | 494 | 100% (Omnipresent) |
| Ardea purpurea | 01 | 26 | 50% (Constant) |
| Ardea alba | 04 | 08 | 42.85% (Common) |
| Nycticorax nycticorax | 05 | 19 | 35.71% (Common) |
| Ardeola ralloides | 03 | 21 | 50% (Constant) |
Table 3. Frequency of occurrence (C%) of Ardeidae species recorded at Lac Tonga.
Recorded species have different frequencies of occurrence (14 classes). The most frequent species were the Western Cattle egret and Little egret, with a value of 100% during the study period. These results can be explained by their high numbers and distribution on several feeding sites. The constant species are the Grey heron with 64.28%, the Purple heron and the Squacco heron with 50%. The common species are the Great egret, with 42.85% and the Night heron, with 35.71%, during the study period.
The evolution of the Ardeidae Equitability Index chart on the site is similar to the Shannon Weaver Diversity Index. The diversity of Shannon weaver and the equitability index during the study period varied during the wintering and breeding seasons, depending on the richness and abundance of Ardeidae species at Lac Tonga. However, it generally showed a diversified population. The first one displayed a value greater than 0.4 bits, and the second showed values greater than 0.3 over the study period. H’max exhibited a value equal to 1.94. Generally, the lowest values were recorded during the second half of March. Minimum values were E=0.35 and H’=0.49, with a less unbalanced population. The maximum ones were recorded during the second half of June with H’=1.4 and E=0.88, corresponding to a balanced abundance distribution. These findings indicate that breeding species mainly dominate the Ardeidae stand (Fig. 7).
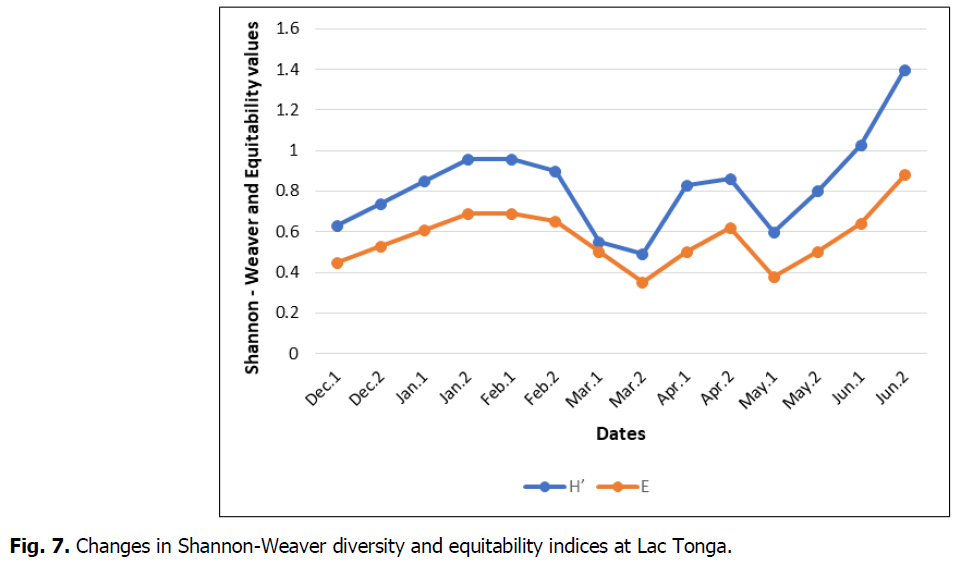
Fig 7: Changes in Shannon-Weaver diversity and equitability indices at Lac Tonga.
During our counts, we tried to specify the habitat type used by the Ardeidae inventoried at Lac Tonga to analyze each species’ spatial distribution. The Western Cattle heron is a very frequent species in the northeastern region of Algeria and precisely at Lac Tonga. It is represented with quite high numbers during the study period. It is also observed in the formation of Alder (Alnus) and the common water-crowfoot (Ranunculus aquatilis) on the banks of the lake (Fig. 8). Furthermore, the results indicate that Little egrets and Western Cattle egrets occupy Lac Tonga more or less similarly. However, Little egrets use open water and areas with floating vegetation: branched bur-reed (Sparganium erectum) and the lakeshore bulrush or common club-rush (Schoenoplectus lacustris) much more. Both populations are generally concentrated in the northeastern part and around the lake’s shoreline (Fig. 8).
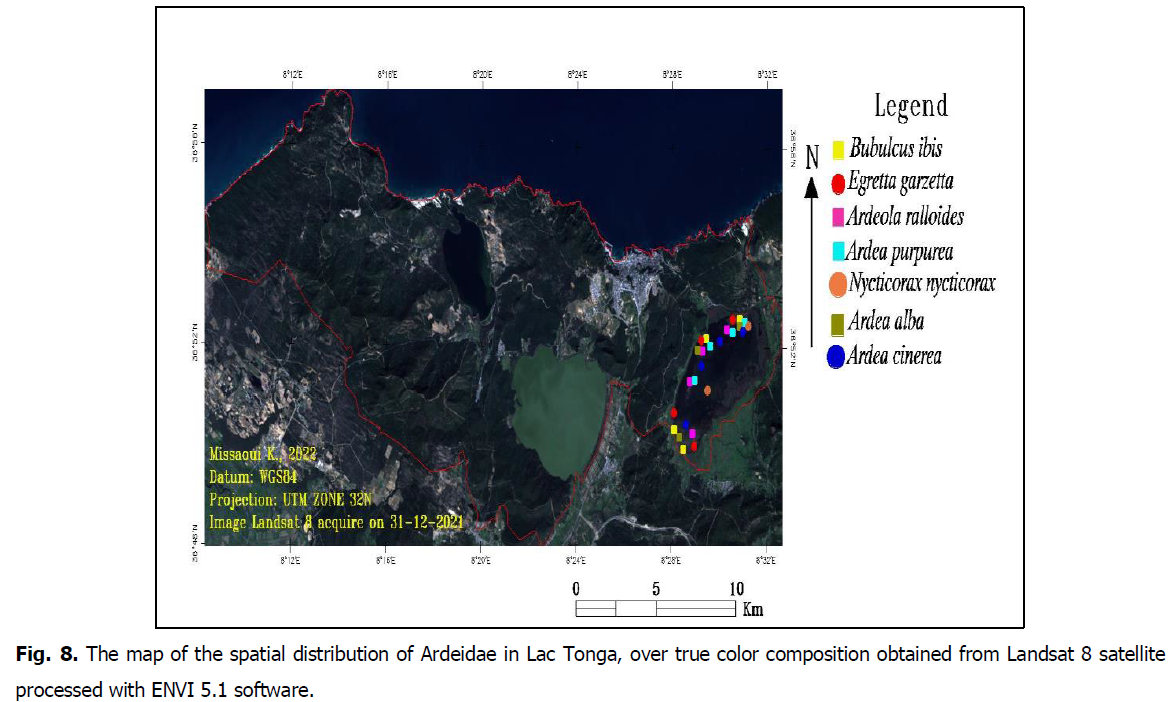
Fig 8: The map of the spatial distribution of Ardeidae in Lac Tonga, over true color composition obtained from Landsat 8 satellite processed with ENVI 5.1 software.
The observations show that the Night heron and Squacco heron frequent the wetland banks. The first species is more concentrated on the northeastern shoreline at the common club-rush (Schoenoplectus lacustris) and was mostly observed in the center of the water body (Fig. 8). In contrast, the second has a wider distribution. It can be observed on the south, west and northeast shores at the level of the branched bur-reed and the common reed at the level of the branched bur-reed and the common reed (Phragmites australis) and sometimes inside the lake on the stems of rushes (Fig. 8). Purple heron primarily exploits the northern and northwestern portions of the lake, and this significant occupancy is observed from March to May. Moreover, it exploits the phragmites of the northern part near the National Park Surveillance Brigade and the western part of the lake near the watchtower at the branched bur-reed and the common club-rush (Fig. 8).
The Great egret was observed during the wintering period with low numbers. The population is concentrated on the northern and western shores of the lake, at a depth of 1 m, where it dominates the emergent willow (Salix) vegetation (Fig. 8). The Grey heron is found in the shallow areas of the lake. Its presence extends along the northern and southern shores and the western part near the watchtower. It exploits much more branched bur-reed (Sparganium erectum) (Fig. 8). This wading bird is sometimes found near agricultural lands to hunt rodents and other terrestrial prey.
Discussion
This study aims to expand some of the research conducted in Algeria on Ardeidae’s habitat and ecological niche. The Eastern Numidia region contains many special wetlands because of their biodiversity and variety. Several scientific studies have been carried out in this area (Samraoui and De Belair, 1998). Chalabi (2008) reported that the great majority of the Heron colonies of Algerian Numidia are often observed in the wetland complex of El Kala, notably Lac Tonga, Garaet Eddakhla and Garaet Stah. We also mention the wetlands of the eco-complex of Setif and Guerbes-Sanhaja (Skikda) (Baaziz, et al., 2011; Metallaoui, et al., 2014). In these last two regions, Ardeidae are represented by 8 species, including the Western Cattle egret, which is the most represented species with 350 individuals in Setif (Baaziz, et al., 2011) and 810 individuals in Skikda (Metallaoui, et al., 2014), followed by the Little egret with 17 and 260 individuals respectively.
The other six species are less represented in both regions (Baaziz, et al., 2011; Metallaoui, et al., 2014). Echatt Marsh is home to the nesting of many species of birds during the breeding season, such as the Purple heron, the Western Cattle heron, the Little egret, the Little grebe and the Ferruginous Duck. Some are vulnerable and endangered (Chettibi, et al., 2014). The wetlands of Souk Ahras region host four species of Ardeidae, including the Western Cattle egret, which is the most represented in the three water bodies (Tiffech dam, El Kef dam and Medjen Djedj marsh), with 275 individuals. However, the Great egret, the Little egret and the Grey heron are represented with low numbers (Guellati, et al., 2014).
Ardeidae count at Boussedra Marsh (Annaba) showed that the maximum number of the Western Cattle heron reached 2500 individuals in 2014, whereas the Squacco heron reached 594 individuals (Boudraa, et al., 2014). This last species breeds from April to July in Eurasia and North Africa. The Sahara south populations breed mainly during the rainy season (Chedad, et al., 2022; Del Hoyo, et al., 1992). After the breeding season, Palearctic populations migrate south and usually winter in tropical Africa from August to November (Del Hoyo, et al., 1992; Kushlan and Hancock, 2005; Benyacoub, et al., 2011; Birdlife International, 2018) to return to the breeding colonies in the Mediterranean regions between February and May (Kushlan and Hancock, 2005; Birdlife International, 2018). However, the number of Little bittern Ixobrychus minutus at Boussedra Marsh ranged from 11 to 33 individuals (Boudraa, et al., 2014).
Outside the Numidia region, Gareat Boughrara (Tlemcen) is home to a monospecific colony of the Western Cattle heron. Moreover, the Night heron and the Squacco heron have been observed outside the colony (Samraoui-Chenafi, 2009). The presence of a hundred Western Cattle egret and twenty Night heron on a stratum of Scripus triqueter at Gareat Timerganine (Samraoui-Chenafi, 2009; Seddik et al., 2010), also it is a breeding site for Little bittern and Squacco heron (Samraoui, et al., 2011). The Bougara Dam is an ideal nesting site for many Ardeidae species, such as the Squacco heron, the Western Cattle egret, the Night heron, and the Grey heron (Meziane, et al., 2022). The Little egret is observed during the winter season and passage throughout the country, including the Sahara; Ouargla, Touggourt (Lac Tamacine), Biskra (Outaya), Ghardaïa and El Ménéa (Kef Doukhane, Oued Bir, Hassi Ghanem) (Isenmann and Moali, 2000; Samraoui and Samraoui, 2008; Samraoui, et al. 2011; Chedad, et al., 2020b; Chedad, 2021). The island of Rachgoun (Ain Temouchent) hosts a mixed colony of Western Cattle heron and the Little egret nests on a Cliffs flank (Samraoui-Chenafi, 2009).
Several studies have shown that the spatial distribution of waterbirds varies over time, depending on the biological and ecological criteria of the species, in addition to the environmental conditions of their surroundings (Houhamdi and Samraoui, 2008; Metallaoui, et al., 2009). Tranquility and sharing of trophic resources play some role in the distribution of waterbirds on the water body (Houhamdi and Samraoui, 2008; Metallaoui et al., 2009; Elafri et al., 2017). At Boussedra Marsh, the Western Cattle heron, the Squacco heron, the Night heron and the Little egret nest on the lake’s western shore and exactly Tamarix Gallica (Boudraa, et al., 2014). The Western Cattle heron regularly frequents Gareat Timerganine but does not nest there. However, the Little egret breeds there. This lake is also an ideal wintering site for many rare or endangered water species in the Western Palearctic and a privileged breeding site for the Night heron and the Little bittern (Seddik, et al., 2010). At the level of the eco-complex of Setif, the Western Cattle heron breeds in the trees of White Poplar Populus Alba and Aleppo pine, Pinus halpensis, near the White storks Ciconia ciconia (Baaziz, et al., 2011).
The avian diversity in Lac Tonga is also important. It is a wintering and reproduction ground for other waterbirds. The literature reports that 35 species visited this humid zone, and 17 were nested. Examples include Tachybaptus ruficollis, Podiceps crystatus, Porphyrio porphyrio, Anas platyrhynchos, and Aythya nyroca, some of which are important internationally (Rizi, 2018; Loucif, 2020; Gherib, et al., 2021).
Conclusion
This study aimed to clarify and enrich our knowledge of the Lac Tonga population’s structure, dynamics, distribution patterns and spatial occupancy. The results of this study indicate the importance of Lac Tonga as a wintering and breeding area for some species of Ardeidae. The data collected, although recent, need to be supplemented by further work on the ecology and breeding biology of these birds, their habitat diversity, and the ethology of some species.
Acknowledgement
In presenting the results of our work, we would like to thank all those who made it possible. This study was supported by the General Directorate of Scientific Research and Technological Development (DGRSDT, Algeria) and the Algerian Ministry of Higher Education and Scientific Research (MESRS, Algeria). The authors are very grateful to these two institutions. Similarly, we are very grateful to the reviewers for their comments and to the editor for the opportunity to review our manuscript.
References
Arendt, W., Batty, C.J. (1988). Tauberian theorems and stability of one-parameter semigroups. Transactions of the American Mathematical Society, 306:837-852.
Baaziz, N., Mayache, B., Saheb, M., Bensaci, E., Ounissi, M., Metallaoui, S., Houhamdi, M. (2011). Phenological status and reproduction of waterbird populations in the Setif wetland eco-complex (High plateaus, eastern Algeria). Bulletin of the Scientific Institute of Rabat, 32:77-87.
Bachelier, G. (1978). La faune des sols: son écologie et son action.
Bakaria, F., Belhaoues, S., Djebbari, N., Tahri, M., Ladjama, I., Bensaad, L. (2018). Metazoan parasites and health state of european eel, Anguilla anguilla (anguilliformes, anguillidae), from Tonga Lake and El Mellah lagoon in the northeast of Algeria. Vestnik Zoologii, 52:279-288.
Google Scholar, Crossref, Indexed at
Benyacoub, S., Baba Hmed, R., Kara, H., Brahmia, Z. (2011). Plan Directeur de Gestion des Sites Ramsar du Parc National d’El Kala. Lac Tonga. Phase 2 Evaluation et Interprétation des Données.
Bigot, L., & Bodot, P. (1973). Contribution to the biocoenotic study of the garrigue with quercus coccifera iii. dynamics of invertebrate zoocoenosis. Life and Environment, 23:251-267.
BirdLife, I. (2018). State of the world’s birds: taking the pulse of the planet.
Blondel, J. (1969). Méthodes de dénombrement des populations d’oiseaux. cité par Lamotte M. et Bourliere F., Problème d’écologie. Ed. Masson et Cie, pp:97-151.
Blondel, J. (1975). Les écosystèmes de Camargue. Le courrier de la Nature, 35:43-56.
Boisteau, B., Marion, L. (2006). Landscape influence on the Grey Herons colonies distribution. Comptes Rendus Biologies, 329:208-216.
Google Scholar, Crossref, Indexed at
Boudraa, W., Bouslama, Z., Houhamdi, M. (2014). Inventaire et écologie des oiseaux d’eau dans le marais de Boussedra (Annaba, nord-est de l’Algérie). Bulletin Social and Zoology in France, 139:279-293.
Boukrouma, N., Maazi, M.C., Saheb, M., Metallaoui, S., Houhamdi, M. (2011). Hivernage du Canard Pilet Anas acuta sur les hauts plateaux de l'Est de l'Algérie. Alauda, 79:285-293.
Chalabi-Belhadj, G. (2008). Contribution à l’étude des exigences écologiques des Ardeirdae et de l’Ibis falcinelle Plegadis falcinellus dans le complexe des zones humides d’El Kala (Algérie).
Chedad, A., Bouzid, A., Samraoui, B. (2022). First successful nesting of the Little Egret Egretta garzetta in Ghardaïa (Algerian Sahara). Zoology and Ecology, 32:68-73.
Abdelwahab, M. C. (2021). Bio-écologie des espèces aviennes dans quelques écosystèmes sahariens (Ghardaïa): Cas du Bruant du Sahara (Doctoral Dissertation, Universite Kasdi Merbah-Ouargla).
Google Scholar, Crossref, Indexed at
Chedad, A., Bendjoudi, D., Guezoul, O. (2020). Biodiversity of the aquatic avifauna of an artificial wetland in Kef Doukhane (Ghardaïa, Algerian Sahara). Bulletin Social and Zoology in France, 145:383-400.
Chedad, A., Bouzid, A., Bendjoudi, D., Guezoul, O. (2022). New observations of four waterbird species in Algerian Sahara. African Journal of Ecology, 60:516-522.
Google Scholar, Crossref, Indexed at
Ahlam, C. (2020). Use of the habitats and distribution factors of certain avian species in the wetlands of North-East Algeria.
Ahlam, C., Ettayib, B., Fateh, M., Soumia, D. (2019). Effects of vegetation and water seasonal variation on habitat use of herons (Aves, Ardeidae) in Tonga Lake (North-East Algeria).
Chettibi, F., Aberkane, M., Draidi, K., Bakhouche, D., Guerguebe, L., Bouslama, Z., Houhamdi, M. (2014). Breeding ecology of water birds in Echatt (Numidia, north-eastern Algeria). Annals of Biological Research, 5:27-31.
Daget, P. (1979). Espèces indicatrices et leur valeur caractéristique vis-à-vis du milieu. Natural Monsp, sér. Bota, 93:107.
Dajoz, R. (1971). Précis d’écologie. Ed. Barda, Paris, p:434.
Belair, G.D. (2005). Dynamics of the vegetation of temporary pools in North Africa (eastern Numidia, NE Algeria). Ecologia Mediterranea , 31:83-100.
Del Hoyo, J., Del Hoyo, J., Elliott, A., Sargatal, J. (1992). Handbook of the birds of the world. Barcelona: Lynx Edicions, 1:8.
Elafri, A., Belhamra, M., Houhamdi, M. (2017). Comparing habitat preferences of a set of waterbird species wintering in coastal wetlands of North Africa: implication for management. Ekológia (bratislava), 36:158-171.
Elafri, A. (2017). Inventory and ecology of the population of aquatic birds in a Ramsar site in northeastern Algeria (Lake Tonga, wilaya El-Tarf).
Fasola, M., Merli, E., Boncompagni, E., Rampa, A. (2011). Monitoring heron populations in Italy, 1972-2010. Journal of Heron Biology and Conservation, 1:1-10.
Faurie, C., Ferra, C., Medori, P., Devot, J., Hemptienne, J.L. (2003). Ecologie. Approche scientifque et pratique, 5ème édition. Edditions TEC and DOC, Paris.
Gherib, A., Lazli, A., Naili, S., Boucheker, A., Ikhlef, D., Mechaka, N.I. (2021). Avifauna diversity and phenology in a Ramsar site: Lake Tonga (Northeastern Algeria). Arxius deMiscellània Zoològica, 19:321-344.
Guellati, K., Maazi, M.C., Benradia, M., Houhamdi, M. (2014). Le peuplement d’oiseaux d’eau du complexe des zones humides de la wilaya de Souk-Ahras: état actuel et intérêt patrimonial. Societe Zoologique de France, p:139.
Houhamdi, M., Samraoui, B. (2001). Diurnal time budget of wintering Teal Anas crecca at Lac des Oiseaux, northeast Algeria. Wildfowl, 52:87-96.
Houhamdi, M., Samraoui, B. (2008). Diurnal and nocturnal behaviour of Ferruginous duck Aythya nyroca at Lac des Oiseaux, northern Algeria. Ardeola, 55:59-69.
Isenmann, P., Moali, A. (2000). Birds of Algeria. Paris Société d’Études Ornithologiques de France.
Kadid, Y. (1989). Contribution à l’étude de la végétation aquatique du lac Tonga-Parc national d’El Kala. Mémoire d’ingénieur, INA El Harrach, Alger.
Kushlan, J.A., Hancock, J. A. (2005). The herons. Ox-ford University Press, Oxford, U.K.
Lamotte, M., Bourlière, F. (Eds.). (1969). Problèmes d'Écologie: l'échantillonnage des peuplements animaux des milieux terrestres. Masson et Cie.
Lazli, A., Boumezbeur, A., Moali-Grine, N., Moali, A. (2011). Evolution of the nesting population of the White-headed Duck Oxyura leucocephala on Lake Tonga (Algeria). Journal of Ecology, Earth and Life, 66:173-181.
Lazli, A., Boumezbeur, A., Perennou, C., Moali, A. (2011). Biologie de la reproduction de l'érismature à tête blanche Oxyura leucocephala au lac Tonga (Algérie). Revue d'Ecologie, Terre et Vie, 66:255-265.
Legendre, L., Legendre, P. (1984). Ecologie numérique: la structure des données écologiques. 2ème éd. Québec, Université du Québec, p:328.
Lindström, A., Piersma, T. (1993). Mass changes in migrating birds: the evidence for fat and protein storage re‐examined. Ibis, 135:70-78.
Loucif, K. (2020). Etude de la qualité physico-chimique et bactériologique de l’eau du Lac Tonga (wilaya d’El Tarf) et occupation spatio-temporelle du site par l’avifaune aquatique.
Marion, L., Duhautois, L. (1986). Effets de la vague de froid de janvier 1985 sur les effectifs reproducteurs du Héron cendré et de l'Aigrette garzette au printemps 1985. Rapport SNPN Laboratoire d'Evolution des Systèmes naturels et Modifiés Ministère de l’Environnement (DPN-SRETIE).
Menasria, B., Lazli, A. (2017). Quelques aspects de la sélection de l’habitat par l’Érismature à tête blanche Oxyura leucocephala sur le Lac Tonga. Alauda, 85:283-294.
Metallaoui, S., Atoussi, S., Merzoug, A., Houhamdi, M. (2009). Hivernage de l’Erismature à tête blanche (Oxyura leucocephala) dans Garaet Hadj-Tahar (Skikda, nord-est de l’Algérie). Aves, 46:136-140.
Metallaoui, S., Maazi, M.C., Saheb, M., Houhamdi, M., Barbraud, C. (2014). A comparative study of the diurnal behaviour of the Northern Shoveller (Anas clypeata) during the wintering season at Garaet Hadj-Tahar (North-East Algeria) and Garaet Timerganine (Algerian highlands). Turkish Journal of Zoology, 38:158-167.
Meziane, B., Taibi, A., Mairif, M. (2022). Biodiversity and nesting success of waterbirds at the Bougara Dam (Tissemsilt, North-West of Algeria). Ukrainian Journal of Ecology, 12:40-50.
Chokri, M.A., Sadoul, N., Medhioub, K., Bechet, A. (2008). Comparative analysis of the avifaunistic richness of the saline of Sfax in the Tunisian and Mediterranean context. Review of Ecology, Earth and Life, 63:351-369.
Nouri, N., Bahroun, S., Lazli, A. (2022). Écologie trophique de la Foulque macroule Fulica atra dans la réserve intégrale du Lac Tonga (Nord-est algérien). Bulletin de la Société Zoologique de France 2022, 147:69-80.
Pielou, E.C. (1969). An introduction to mathematical ecology. New York, USA, Wiley-Inter-Science.
Ramade, F. (1984). Elément d’écologie. Ecologie fondamental Ed. Mc. Geauw-Hill. Paris, p:397.
Rizi, H. (2018). Etude de l’importance méditerranéenne du complexe des zones humides de la région du Nord à travers l’avifaune hivernante et nicheuse: Cas du Lac Tonga. PhD Thesis, Badji-Mokhtar University, Annaba.
Saifouni, A. (2009). Inventory of wetlands and waterbirds in Algeria.
Saifouni, A., Bellatreche, M., Chebouti-Meziou, N. (2020). Identification et cartographie des habitats naturels du lac tonga (El-kala, Algérie). Revue Agrobiologia, 10:1999-2009.
Samraoui, B., Samraoui, F. (2013). An ornithological survey of Algerian wetlands: Important bird areas, ramsar sites and threatened species. Wildfowl, 58:71-96.
Samraoui, B., De Belair, G. (1998). Les zones humides de la Numidie orientale: bilan des connaissances et perspectives de gestion. Synthèse, 4:1-90.
Samraoui, B., Ouldjaoui, A., Boulkhssaïm, M., Houhamdi, M., Saheb, M., Béchet, A. (2006). The first recorded reproduction of the Greater Flamingo Phoenicopterus roseus in Algeria: Behavioural and ecological aspects. Ostrich-Journal of African Ornithology, 77:153-159.
Samraoui, F., Alfarhan, A.H., Al-Rasheid, K.A., Samraoui, B. (2011). An appraisal of the status and distribution of waterbirds of Algeria: indicators of global changes?. Ardeola, 58:137-163.
Sarri, D. (2017). Développement durable au sein des aires protégées algériennes, cas du Parc National d’EL Kala et des sites d’intérêts biologique et écologique de la région d’El-Tarf. Ferhat Abbas University, Sétif.
Seddik, S., Maazi, M.C., Hafid, H., Saheb, M., Mayache, B., Houhamdi, M. (2010). Statut et écologie des peuplements Laro-Limicoles et Echassiers dans les zones humides des hauts plateaux de l’Est de l’Algérie. Bulletin de l’Institut Scientifique de Rabat, 32:111-118.
Telailia, S., Boutabia, L., Beddak, M. (2018). Première observation de l'aigrette à gorge blanche Egretta gularis en Algérie. First record of the Western Reef-egret Egretta gularis in Algeria. Alauda, 86:319-320.
Author Info
L. Ouarti1*, N. Nouri1, A. Lazli1, K. Missaoui2 and M. Houhamdi32Laboratory Urban Project, City and Territory, Faculty Architecture and Earth Sciences, University of Ferhat Abbas Setif 1. El Bez, Sétif 19000, Algeria
3The Laboratory of Biology Water and Environment, University 8 Mai 1945 Guelma, BP 401, Guelma 24000, Algeria
Citation: Ouarti, L., Nouri, N., Lazli, A., Missaoui, K., Houhamdi, M. (2023). Phenology and spatio-temporal distribution of Ardeidae in Lac Tonga (North-eastern Algeria). Ukrainian Journal of Ecology. 13: 37-49.
Received: 14-Jan-2023, Manuscript No. UJE-23-87143; , Pre QC No. P-87143; Editor assigned: 16-Jan-2023, Pre QC No. P-87143; Reviewed: 28-Jan-2023, QC No. Q-87143; Revised: 04-Feb-2023, Manuscript No. R-87143; Published: 13-Feb-2023, DOI: 10.15421/2023_422
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
