Research - (2022) Volume 12, Issue 10
Population status and fruit safety assessment of Elaeagnus rhamnoides (L.) A. Nelson (Elaeagnaceae Lindl.) in Kazakhstan
G.S. Аidarkhanova1*, S.A. Kubentayev2, K.S. Izbastina1,2 and N.A. Bizhanova3Abstract
The article presents the results of studies on the current status of populations and the safety of fruits of Elaeagnus rhamnoides (L.) A. Nelson (E. rhamnoides) growing in Northern and Eastern Kazakhstan. During expeditions in 2017-2018, we identified environmental factors affecting natural phytocenoses of sea buckthorn in the surveyed areas. We have determined the population examined in Northern Kazakhstan (Kokshetau Upland) to be adventive and successfully established during the virgin land development period. The population is stable, intensively dispersing within new habitats. The Eastern Kazakhstan population is in a distressed state, while it is still unclear where the population originated from. The reason for the stress include strong damage of young shoots and leaves by fungal diseases, cattle grazing, and felling. In the areas where sea buckthorns grow, we examined the radiation gamma background within all study sites noted at a level of up to 0.17 μSv/h. The results of atomic absorption spectroscopy showed that the amount of toxicants of Class 1 (cadmium, arsenic, mercury, lead), Class 2 (cobalt, copper), or of weak toxicity (manganese) in sea buckthorn fruits does not exceed the maximum permissible concentration levels in all samples. Gamma-spectrometric analysis of the radionuclides concentration (11.3-14.1 Bq/kg for 90Sr, 3.5-4.7 Bq/kg for 137Cs) in sea buckthorn fruits showed levels below the threshold limit value. Monitoring of food safety of E. rhamnoides fruits in the Northern and Eastern Kazakhstan forests showed the levels of the toxicants studied to be below the maximum permissible levels. The results of the research conducted allow us to recommend sea buckthorn fruits for use in food, medicinal, and economic purposes.
Keywords
Elaeagnus rhamnoides, Hippophae rhamnoides, Kazakhstan, heavy metals, radionuclides, threshold limit value, food safety.
Introduction
Sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson; syn: Hippophae rhamnoides L., family Elaeagnaceae Lindl.) is a valuable plant for the food and pharmaceutical industry due to the concentration of effective biologically active organic components and minerals (Chen et al., 2010, Li and Beveridge 2003, Musayev, 2013, Pokharel et al., 2021). The widespread use of sea buckthorn since ancient times is due to the rich chemical composition of vegetative organs. The fruits of E. rhamnoides are rich in oils, vitamins C and B, provitamin A, a complex of organic acids (malic, tartaric, nicotinic), tannins, proteins, phytosterols, etc. (Trineeva et al., 2013, Yamansarova et al., 2008, Olas, 2018 a, Gradt et al., 2017). A number of studies has focused on the sea buckthorn’s mineral composition, and determined the concentrations of essential (Fe, Mn, Zn, Cu, Cr, Co, Mo, Se) and toxic (Pb) elements in plant’s fruits, leaves, roots, and bark (Skuridin et al., 2017, Górnaś et al., 2016, Trineeva et al., 2015). Considerable research has been carried out in the direction of biological effects of the various components of E. rhamnoides. It was found that phenolic compounds and a number of organic acids of sea buckthorn fruits have prominent anti-inflammatory, anti-stress, antitumor, antithrombotic, antioxidant properties and lead to the normalization of metabolic processes in the body (Dianlong et al., 2016, Erhan et al., 2017, Koyama et al., 2009, Skalski et al., 2019, Zeb, 2006, Basu et al., 2007, Kumar and Chandrashekar 2011). Due to the high concentration of biologically active compounds and antioxidants, sea buckthorn is included in cancer therapy due to its radioprotective activity (Goel et al., 2002, Goel, et al., 2003, Olas et al., 2018 b). Triterpenoids in the composition of organic substances in sea buckthorn fruits have a hypoglycemic effect, especially when modeling type II diabetes (Lehtonen et al., 2010). The biologically active substances of sea buckthorn have radioprotective properties and are widely used in medical practice (Shi et al., 2017). In addition to sea buckthorn berries, the leaves of this plant, both fresh and dried, are commonly used. They contain a large amount of nutrients and biologically active compounds, including phenolic compounds (Christaki, 2012). Sea buckthorn leaf extracts are used as radioprotective, anti-inflammatory and immunomodulatory substances (Upadhyay et al., 2010, Lee et al., 2011), while sea buckthorn twigs have medicinal properties, as well (Sadowska et al., 2017, Pichiah et al., 2012). With the increase of the anthropogenic disturbance of the natural habitats of sea buckthorn phytocenoses, there are data on the accumulation of heavy metals and radionuclides in the fruits and other organs of E. rhamnoides (Тoropova and Khovalyg 2014, Afanasyeva and Kashin 2015).
The plant is widespread throughout Europe, from England in the north to the Danube in the south, in Asia Minor, Iran, the Himalayas, Tibet, and Mongolia. In Russia, E. rhamnoides occurs in the European part, southern Siberia, Altai and Transbaikal regions (Zhengyi et al., 2007, Gorshkova, 1949, Li and Hu 2015).
Considerable territories of natural phytocenoses of E. rhamnoides in Siberia are found in Tyva, Buryatia, and Altai. In the countries of Central Asia, they are established in the mountainous forests (Panteleyeva, 2006). In Kazakhstan, this species grows in the East in the Kazakh part of Altai; in the Northern and Central regions on the Kokshetau Upland of the Central Kazakhstan Hummocks; as well as in the mountainous regions of the Southern and Southeastern part of the country (Kubentayev et al., 2019, Sultangazina et al., 2014, Pavlov, 1956, Akiyanova et al., 2019).
The aim of this research was to study the current state of the common sea buckthorn populations in natural habitats, and to determine the contamination level of fruits with heavy metals and radionuclides in different regions of Kazakhstan to assess their food safety.
Materials and Methods
The research objects were the fruits of wild-growing sea buckthorn, selected during ripening and mass harvesting by the local people in the regions of Northern and Eastern Kazakhstan. We collected the materials during expedition in 2017-2018. In the field, we studied the geobotanical features of E. rhamnoides phytocenoses using the route method, and determined the resource reserves for harvesting.
When describing plant communities using materials obtained, we applied geobotanical methods with a visual assessment of the number of individuals according to G. Drude’s scale (Bykov, 1970). We analyzed the structure of each specific cenopopulation according to the methods of T.A. Rabotny (Rabotnov, 1964) and O.V. Smirnova (Smirnova, 1976). The methodological instructions developed by M.F. Golubev and E.F. Molchanov were taken as the basis for the study of the ecological and biological characteristics of the species in the field (Golubev and Molchanov 1978). In order to assess the degree of variation of the characteristics studied, we used the coefficient of variation Cυ. We carried out the statistical processing of the material according to the recommendations of G.N. Zaitsev (Zaitsev, 1973). The study of the morphometric parameters of the aerial parts and bulbs in both populations was carried out in 20 repetitions in individuals of the middle-aged generative state. The nomenclature of plant species was verified according to The Plant List (The Plant List, 2013); when describing the communities after the first mention, the author’s quotes of the species were not indicated.
For the assessment of the ecological state of the natural environment, we examined the levels of natural radiation background. In laboratory conditions, we determined the concentration of heavy metals (cadmium, arsenic, mercury, lead, cobalt, copper, manganese) by atomic absorption spectroscopy in accordance with the “State standard of the State system of technical regulation of the Republic of Kazakhstan (ST RK GOST) R 51301-05” (ST RK GOST) Gamma-spectrometer “Progress” studied the content of 90Sr, 137Cs in fruits dried to an air-dry state and ashed at temperatures up to 420°C (MUK, 1977). Based on the results obtained, we conducted a comparative analysis of the compliance of fruits with maximum permissible standards-threshold limit values (TLV) (Sanitary norms and rules of the Republic of Kazakhstan 2011) and assessed their food safety. Taking into account the Fisher-Student criterion, the registered changes in indicators were considered reliable at p≤0.05.
Results and Discussion
Based on the results of expeditions in 2017-2018, and the study of herbarium materials of the Altai Botanical Garden, the Institute of Botany and Phytointroduction, Amanzholov East Kazakhstan University, Komarov Botanical Institute, we have found that in the Kazakh Altai, there is one habitat of E. rhamnoides.
We have studied the current state of the specified population of the species in the territory of Kazakh Altai. The surveyed community is located in the East Kazakhstan region, in the Katon-Karagai district, at the foot of the Sarymsakty ridge, in the valley of the Solnechnaya River, in the vicinity of Topkain village (49.185556° N, 85.503611° E) (Fig. 1), at 622 m above sea level. The relief consists of ravine gullies on a leveled area, along the bedrock banks of the Solnechnaya River. The microrelief of the site is heterogeneous, formed by ledges and gullies. The soils are floodplain, meadow chernozems with a significant layer of alluvial humus, with the underlying layer consisting of river gravel. The projective cover of the ground is 85-90%, the layer thickness is 1.5-2 cm.
Fig 1: Schematic map of the surveyed population of E. rhamnoides in Eastern Kazakhstan (Kazakh Altai) and Northern Kazakhstan (Kokshetau Upland).
The vegetation of the surveyed area is polydominant, represented in the form of herb-grass-shrub associations. The community with the common sea buckthorn is located by a narrow band on both sides of the Solnechnaya River, the width of the band is 25-35 m. In this population, one phytocenosis has been isolated and described. The total projective cover of the site is 85-90%.
Cenopopulation of forbs-reed-sea buckthorn (E. rhamnoides, Calamagrostis epigejos (L.) Roth, Filipendula ulmaria (L.) Maxim., Sanguisorba officinalis L., Achillea millefolium L.) phytocenosis, where E. rhamnoides dominates among shrubs-copiosae2 (cop2-abundant); among the secondary species, Spiraea hypericifolia L.-sp, Cotoneaster melanocarpus Fisch. ex A. Blytt-sol, Rosa acicularis Lindl.-sp, Spiraea chamaedryfolia L.-sol. The density of the shrub layer is 04. The site is located under the canopy of a thinned stand of Betula pendula Roth and Populus tremula L. stands with a fullness of 03.
The herbage has a two-layered structure, the community is dominated by mesophytes and hygromesophytes. In the first layer, the dominant species are Calamagrostis epigeios-cop1, Filipendula ulmaria-cop2, Sanguisorba officinalis-cop3. Among secondary species there can be commonly found Bromus inermis Leyss-sp, Artemisia vulgaris L.-sp, Urtica dioica L., Dactylis glomerata L.-sp, Libanotis buchtormensis (Fisch.) DC.-sol, Angelica sylvestris L.-sol, etc. The height of the first layer is 120-150 cm. The second layer is dominated by Achillea millefolium and the vegetative mass of Calamagrostis epigeios with a cover of up to 30% of the total. Secondary species include Elymus repens (L.) Gould-sol, Rhinanthus alectorolophus (Scop.) Pollich-sp, Inula salicina L.-sol, Medicago falcata L.-sol, Galatella sedifolia (L.) Greuter-sp, Galium verum L.-sp, Stipa capillata L.-sol, Artemisia austriaca Jacq.-sp, Fragaria viridis Weston-sol, Rubus saxatilis L., Vicia cracca L., etc.
The herbage has a two-layered structure, the community is dominated by mesophytes and hygromesophytes. In the first layer, the dominant species are Calamagrostis epigeios-cop1, Filipendula ulmaria-cop2, Sanguisorba officinalis-cop3. Among secondary species there can be commonly found Bromus inermis Leyss-sp, Artemisia vulgaris L.-sp, Urtica dioica L., Dactylis glomerata L.-sp, Libanotis buchtormensis (Fisch.) DC.-sol, Angelica sylvestris L.-sol, etc. The height of the first layer is 120-150 cm. The second layer is dominated by Achillea millefolium and the vegetative mass of Calamagrostis epigeios with a cover of up to 30% of the total. Secondary species include Elymus repens (L.) Gould-sol, Rhinanthus alectorolophus (Scop.) Pollich-sp, Inula salicina L.-sol, Medicago falcata L.-sol, Galatella sedifolia (L.) Greuter-sp, Galium verum L.-sp, Stipa capillata L.-sol, Artemisia austriaca Jacq.-sp, Fragaria viridis Weston-sol, Rubus saxatilis L., Vicia cracca L., etc.
Plants of E. rhamnoides in the surveyed population are in an extremely degraded state. Fruiting is weak, the yield is 25.2 ± 1.07 kg/ha, the operational reserve is 0.3 centners. The damage of fungal diseases on young shoots and leaves is prominent. The population is regressing, with a strong drying of the shoots, and loose bushes. The total area of the cenopopulation is about 1.2 hectares. The number of adult plants is 0.08 ± 0.02 ind./m2. The height of adult plants is 212 ± 5.6 cm. The annual growth is 14.52 ± 1.5 cm.
The distress and consequent degradation of the population can be caused by the presence of a complex of limiting factors, which include natural historical factors, erosion of the banks of the Solnechnaya River, lack of fruiting, severe fungal infection, cattle grazing, felling.
Another population of sea buckthorn was surveyed on the northeastern shore of Shortankol Lake, which is located northwest of Shchuchinsk city, in the Burabay district of Akmola region, Republic of Kazakhstan (53.00577° N, 70.19545° E) (Fig. 1), and located at 359 m above sea level. This territory belongs to the Kokshetau Upland (Karamysheva and Rachkovskaya 1973). The lake is included in the group of Kokshetau lakes and is located within the territory of the Burabay State National Natural Park, Barmashy (Barmashinsky) forestry. The relief is heterogeneous, slightly wavy, ledge. The soils are meadow and boggy chernozems; closer to the water area, narrow lakeside strips of fluvisols are formed.
Sea buckthorn is a part of thickened tree-shrub formations with plantation density 09-1. The surveyed population covers an area of about 2.5 hectares and is located in a narrow strip of 50-70 m along the coastline, where the degree of moisture increases significantly. We examined one cenopopulation in this area.
Cenopopulation of willow-sea buckthorn (E. rhamnoides, Salix caprea L., Salix cinerea L.) phytocenosis with the tree plantations of Betula pendula-sol, Populus laurifolia Ledeb.-sol, Populus nigra L.-r, Pinus sylvestris L.-sol is present. The total projective cover of the area studied is 95-100%. The share of sea buckthorn in the cover accounts for about 75% of the total. The cenopopulation is dominated by E. rhamnoides-soc, Salix caprea-cop1, S. cinerea-cop1-sp. In the lower layer, shrubs of Rosa acicularis-sol, Spiraea hypericifolia L.-sol, Grossularia acicularis (Sm.) Spach-sol are rare.
Herbaceous vegetation is formed mainly at the border of tree-shrub thickets and in open areas. The layering in the grass stand is not expressed. The community is dominated by meso-xerophytic species-Tussilago farfara L.-cop2, Calamagrostis epigeios-cop1, Echium vulgare L.-cop3, Artemisia absinthium L.-sp. Among the secondary species in the phytocenosis, there are Gypsophila altissima L.-sol, Potentilla chrysantha (Zoll. and Moritzi) Trevir.-sp, Silene nutans L.-sol, Artemisia sericea Weber ex Stechm.-sp, Setaria viridis (L.) P. Beauv.-sp, Tanacetum vulgare L.-sp, Artemisia marschalliana Spreng.-sol, Achillea millifolium-sol, Fragaria vesca L.-sp, etc.
The vegetation of the area studied has undergone significant anthropogenic transformation. This is evidenced by the presence of a large number of adventive and ruderal plants in the community (Populus laurifolia, P. nigra, Grossularia acicularis, Setaria viridis, Echium vulgare, Tussilago farfara, Hippophae rhamnoides, etc.). The reason can be the close proximity to the city of Shchuchinsk, seasonal recreational load, naturalization of some species from city parks, squares and nursery-gardens.
According to the literature (Kumar and Chandrashekar 2011, Goel et al., 2003) and the results of our own research, we have found that E. rhamnoides in the surveyed area is an adventive species that successfully naturalized during the development of virgin lands. In the flora of Kazakhstan and in other sources (Kumar and Chandrashekar 2011, Goel et al., 2003) E. rhamnoides was not recorded on the territory of the Kokshetau Upland until 1980, which confirms the anthropogenic origin of the species in this territory. The surveyed sea buckthorn community has plants of different ages, there is vegetative and seed regeneration. The cenopopulation is dominated by female plants. Abundant fruiting is noted, where we have noted an average yield of 87.2 ± 3.07 kg/ha, the volume of possible annual harvesting will be 1.9-2.0 centners with an operational reserve of 2.66 centners. Plant height varies within 270 ± 5.6 cm. The number of adult plants is estimated at 0.75 ± 0.03 ind./m2. The annual growth is 21 ± 0.8 cm. The general state of the population is progressive,-it is intensively settling, capturing new habitats.
In general, areas of natural vegetation in the north and east of the republic have long been intensively developed by industrial enterprises in the extraction of metal ores, by individual entrepreneurs, especially in the eastern part of the country. As a result of this activity, the components of the natural environment, especially the soil and vegetation cover, are contaminated with biologically toxic elements as a result of wind-dust transfer, with water flows, forming local foci of pollution. These processes require the organization of environmental monitoring to control radiation and toxicological parameters near polymetallic and uranium mines, agricultural and recreational areas. The analysis of the data on food safety is presented in Table 1 and Fig. 2.
| Sampling location | Heavy metals, mg/kg | |||||
|---|---|---|---|---|---|---|
| Most toxic Class 1 |
Toxic/Moderately toxic Class 2 |
Slightly toxic Class 3 |
||||
| Cd | As | Pb | Co | Cu | Mn | |
| Northern Kazakhstan | 0.004 ± 0.0007 | 0.002 ± 0.0001 | 0.03 ± 0.006 | 0.027 ± 0.003 | 3.8 ± 0.5 | 3.1 ± 0.8 |
| Eastern Kazakhstan | 0.004 ± 0.0006 | 0.002 ± 0.0003 | 0.03 ± 0.005 | 0.021 ± 0.005 | 4.3 ± 0.9 | 2.3 ± 0.5 |
| TLV | 0.03 | 0.2 | 0.4 | - | 5.0 | - |
Table 1. Results of studies of contamination of E. rhamnoides fruits with heavy metals and radionuclides.
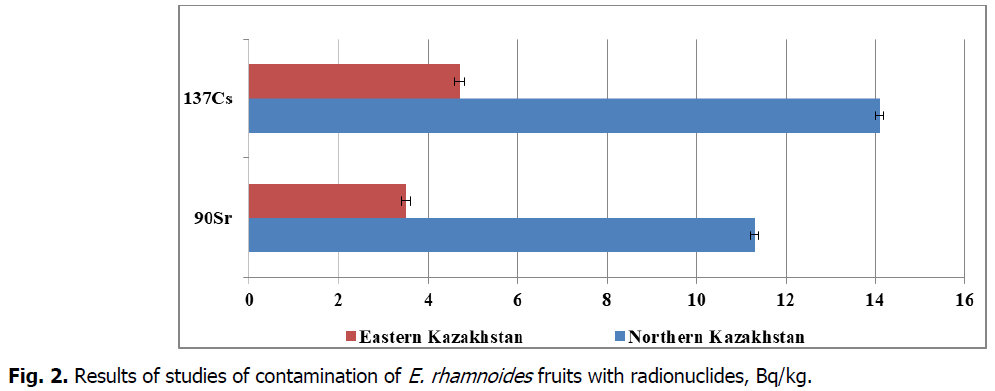
Fig 2: Results of studies of contamination of E. rhamnoides fruits with radionuclides, Bq/kg.
The determined concentrations of cadmium and arsenic are in the ranges below the TLV from 12 to 90 times. In some experiments, it was shown that the concentration of manganese in ripe fruits of sea buckthorn does not exceed the level of 3.0 mg/kg (Pokharel et al., 2021, Yamansarova et al., 2008).
In accordance with the regulatory documents, all metals studied are ranked into groups according to their degree of hazard: most toxic (Class 1), moderately toxic (Class 2), slightly toxic (Class 3). Carrying out a comparative analysis with permissible values showed that of all the elements studied, the concentrations of copper and lead tend to the upper level of the permissible limits, without exceeding it.
From the results of our research, the concentration of manganese is in the range of 2.3-3.1 mg/kg. If we take into account the fact that manganese as a source of an essential element for the human body, then its concentration in sea buckthorn fruits is sufficient for the daily consumption rate. According to the research results, it might be stated that the concentrations of heavy metals, especially toxic elements of the first class, will not have a negative effect when sea buckthorn fruits are used for food purposes.
We studied the specific activity of radionuclides in sea buckthorn fruits due to the fact that there are operating uranium mines on the territory of Kazakhstan, both in the northern and eastern regions. Furthermore, it is well known that long-term nuclear tests were carried out in the country, resulting in formation of local radioactive contamination areas. Analysis of the radionuclide contamination of sea buckthorn fruits showed that the 90Sr content varies within the range of 11.3-14.1 Bq/kg, and the 137Cs content is in the range of 3.5-4.7 Bq/kg. Comparison of all the established values with the threshold limit values showed that the 90Sr content is 11-14 times lower than the TLV, while the 137Cs content is 12-17 times lower than the TLV in all samples of wild-growing sea buckthorn fruits.
There were also no significant differences in the concentration of heavy metals and radionuclides in sea buckthorn fruits depending on the region of the survey. In general, the differences are noted at the level of measurement errors. All this allows us to state that the levels of contamination of sea buckthorn fruits from different regions of Kazakhstan with heavy metals and radionuclides do not exceed the threshold limit values.
The research was conducted within the framework of the Grant Financing Project of the Ministry of Education and Science of the Republic of Kazakhstan No. AP05136154 for 2018-2020.
Conclusion
Natural populations of the wild-growing sea buckthorn E. rhamnoides in different regions of Kazakhstan have differences in general condition. Phytocenoses of sea buckthorn in the eastern part of the country are in a degraded state due to irrational use by local residents (excessive felling, overcollection of sea buckthorn plants and berries, grazing of animals) and habitat changes (erosion of the coastal part of water bodies, the spread of fungal diseases). In the northern regions, the population ranges are expanding due to the intensive dispersal of plants under favorable natural and climatic conditions.
The contamination of sea buckthorn fruits with heavy metals is noted to be below the level of threshold limit values for all elements studied (cadmium, arsenic, mercury, lead, cobalt, copper, manganese), which enables recommendation of their rational collection and preparation for practical use in the surveyed territory of Kazakhstan.
The levels of radionuclide contamination of sea buckthorn fruits (11.3-14.1 Bq/kg for 90Sr; and 3.5-4.7 Bq/kg for 137Cs) in various regions of Kazakhstan do not exceed permissible levels according to the State sanitary and hygienic standards.
The results of the research performed are used to improve the level of ecological education of the populace on the rational use of non-timber forest resources. Studies of the quality of wild-growing sea buckthorn fruits in the field of food safety allow us to recommend their widespread use for food, medical, economic purposes and harvesting in industrial volumes.
References
Afanasyeva, L.V., Kashin, V.K. (2015). Heavy metals accumulation in the berries of hippophae rhamnoides (elaeagnaceae) under the influence of traffic pollution (republic of buryatia). Rastitel'nye Resursy, 51:554-563.
Akiyanova, F., Atalikhova, A., Jussupova, Z., Simbatova, A., Nazhbiev, A. (2019). Current state of ecosystems and their recreational use of the Burabai National Park (Northern Kazakhstan). Eurasian Journal of Biosciences.
Basu, M., Prasad, R., Jayamurthy, P., Pal, K., Arumughan, C., Sawhney, R.C. (2007). Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine, 14:770-777.
Google Scholar, Crossref, Indexed at
Bykov, B.A. (1970). Introduction to phytocenology. Alma-Ata (in Russian).
Chen, W., Su, X., Zhang, H., Sun, K., Ma, R., Chen, X. (2010). High genetic differentiation of Hippophae rhamnoides ssp. yunnanensis (Elaeagnaceae), a plant endemic to the Qinghai-Tibet Plateau. Biochemical Genetics, 48:565-576.
Google Scholar, Crossref, Indexed at
Christaki, E. (2012). Hippophae rhamnoides L. (Sea Buckthorn): a potential source of nutraceuticals. Food Public Health, 2:69-72.
Diandong, H., Feng, G., Zaifu, L., Helland, T., Weixin, F., Liping, C. (2016). Sea buckthorn (Hippophae rhamnoides L.) oil protects against chronic stress-induced inhibitory function of natural killer cells in rats. International Journal of Immunopathology and Pharmacology, 29:76-83.
Google Scholar, Crossref, Indexed at
Erhan, E., Terzi, S., Celiker, M., Yarali, O., Cankaya, M., Cimen, F.K., Suleyman, B. (2017). Effect of Hippophae rhamnoides extract on oxidative oropharyngeal mucosal damage induced in rats using methotrexate. Clinical and Experimental Otorhinolaryngology, 10:181.
Google Scholar, Crossref, Indexed at
Gradt, I., Kuhn, S., Morsel, J., Zvaigzne, G. (2017). Chemical composition of sea buckthorn leaves, branches and bark. Proceedings of the Latvian Academy of Sciences, 3:211-216.
Goel, H.C., Prasad, J., Singh, S., Sagar, R.K., Kumar, I.P., Sinha, A.K. (2002). Radioprotection by a herbal preparation of Hippophae rhamnoides, RH-3, against whole body lethal irradiation in mice. Phytomedicine, 9:15-25.
Google Scholar, Crossref, Indexed at
Goel, H.C., Salin, C.A., Prakash, H. (2003). Protection of jejunal crypts by RH‐3 (a preparation of Hippophae rhamnoides) against lethal whole body gamma irradiation. Phytotherapy Research, 17:222-226.
Google Scholar, Crossref, Indexed at
Golubev, V.N., Molchanov, E.F. (1978). Guidelines for the population-quantitative and ecological-biological study of rare, endangered and endemic plants of the Crimea. Yalta, (In Russian).
Gorshkova, S.G., Lokhovye, E. (1949). Flora SSSR: M.-L.: Izd-vo AN SSSR, 15:515-525 (In Russian).
Górnaś, P., Šnē, E., Siger, A., Segliņa, D. (2016). Sea buckthorn (Hippophae rhamnoides L.) vegetative parts as an unconventional source of lipophilic antioxidants. Saudi Journal of Biological Sciences, 23:512-516.
Google Scholar, Crossref, Indexed at
Karamysheva, Z.V., Rachkovskaya, E.I. (1973). Botanical geography of the steppe part of Central Kazakhstan. Botanical Institute RAS, St. Petersburg.
Koyama, T., Taka, A., Togashi, H. (2009). Effects of a herbal medicine, Hippophae rhamnoides, on cardiovascular functions and coronary microvessels in the spontaneously hypertensive stroke-prone rat. Clinical Hemorheology and Microcirculation, 41:17-26.
Kubantsev, S.A., Kotukhov, Yu.A., Gimadie-va, N.G., Muchabaiwa, S.K. (2011). Current status ofpopulations of rare medicinal plants inthe Kazakhstan Altai. Botanical Researches of Siberia and Kazakhstan, 25:102-111 (In Russian).
Kumar, T., Chandrashekar, K.S. (2011). Bauhinia purpurea Linn.: A review of its ethnobotany, phytochemical and pharmacological profile. Research Journal of Medicinal Plant, 5:420-431.
Google Scholar, Crossref, Indexed at
Li, Y., Hu, C. (2015). Hippophae rhamnoides L. sea buckthorn (Shaji, Common Sea-buckthorn). In Dietary Chinese Herbs, Springer, Vienna, pp. 403-415.
Li, T.S., Beveridge, T.H. (2003). Sea buckthorn (Hippophae rhamnoides L.): production and utilization. NRC Research Press.
Lee, H.I., Kim, M.S., Lee, K.M., Park, S.K., Seo, K.I., Kim, H.J., Lee, M.K. (2011). Anti-visceral obesity and antioxidant effects of powdered sea buckthorn (Hippophae rhamnoides L.) leaf tea in diet-induced obese mice. Food and Chemical Toxicology, 49:2370-2376.
Google Scholar, Crossref, Indexed at
Lehtonen, H.M., Järvinen, R., Linderborg, K., Viitanen, M., Venojärvi, M., Alanko, H., Kallio, H. (2010). Postprandial hyperglycemia and insulin response are affected by sea buckthorn (Hippophaë rhamnoides ssp. turkestanica) berry and its ethanol-soluble metabolites. European Journal of Clinical Nutrition, 64:1465-1471.
Google Scholar, Crossref, Indexed at
Musayev, M.K. (2013). Agro-ecological characteristics of sea buckthorn (Hippophae rhamnoides L.) in Azerbaijan. Journal of Crop and Weed, 9:114-120.
MUK. (1977). Radiation monitoring. Strontium-90 and Cesium-137. Food products. Sampling, analysis and hygienic assessment. Methodological guidelines for control methods. Moscow: Nauka (In Russian).
Olas, B. (2018). The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) oil. Journal of Ethnopharmacology, 213:183-190.
Google Scholar, Crossref, Indexed at
Olas, B., Skalski, B., Ulanowska, K. (2018). The anticancer activity of sea buckthorn [Elaeagnus rhamnoides (L.) A. Nelson]. Frontiers in Pharmacology, 9:232.
Google Scholar, Crossref, Indexed at
Panteleyeva, E.I. (2006). Sea buckthorn (Hippophae rhamnoides L.). Barnaul: RAAS. Sib. branch NIIS (In Russian).
Pavlov, N.V. (1956). Flora of Kazakhstan. Alma-Ata: Publishing House of the Academy of Sciences of the Kazakh SSR, 1966.
Pichiah, P.T., Moon, H.J., Park, J.E., Moon, Y.J., Cha, Y.S. (2012). Ethanolic extract of seabuckthorn (Hippophae rhamnoides L) prevents high-fat diet–induced obesity in mice through down-regulation of adipogenic and lipogenic gene expression. Nutrition Research, 32:856-864.
Google Scholar, Crossref, Indexed at
Rabotnov, T. A. (1964). Opredelenie vozrastnogo sostava populyatsiy vidov v soobshchestve. Polevaya geobotanikа [Definition of age structure of species populations in the community. Field geobotany] Moscow-Leningrad.
Sadowska, B., Budzyńska, A., Stochmal, A., Żuchowski, J., Różalska, B. (2017). Novel properties of Hippophae rhamnoides L. twig and leaf extracts-anti-virulence action and synergy with antifungals studied in vitro on Candida spp. model. Microbial Pathogenesis, 107:372-379.
Google Scholar, Crossref, Indexed at
Astana. (2011). Sanitary norms and rules of the Republic of Kazakhstan, No. 611. Order of the Ministry of Health of the Republic of Kazakhstan.
Shi, J., Wang, L., Lu, Y., Ji, Y., Wang, Y., Dong, K., Sun, W. (2017). Protective effects of seabuckthorn pulp and seed oils against radiation-induced acute intestinal injury. Journal of Radiation Research, 58:24-32.
Skalski, B., Kontek, B., Lis, B., Olas, B., Grabarczyk, Ł., Stochmal, A., Żuchowski, J. (2019). Biological properties of Elaeagnus rhamnoides (L.) A. Nelson twig and leaf extracts. BMC Complementary and Alternative Medicine, 19:1-12.
Skuridin, G.M., Chankina, O.V., Baginskaya, N.V. (2017). Accumulation of essential elements and lead in fruits and vegetative parts of sea buckthorn. Chemistry of Vegetable Raw Materials, 1:113-117.
Smirnova, O.V. (1976). The volume of the counting unit in the study of coenopopulations of plants of various biomorphs. Plant Pricing: Basic Concepts and Structure, pp:72-80.
Sultangazina, G.K., Kuprijanov, A.N., Adekenov, S.M. (2014). Flora of the" Burabay" national natural park. Novosibirsk: Publishing House of the SB RAS.
http://www.theplantlist.org/
Тoropova, E.Y., Khovalyg, N.А. (2014). Ecological assessment of habitats and fruits of sea-buckthorn in Republic Tyva. Fundamental Research, 11:1732-1735.
Trineeva, O.V., Safonova, I.I., Safonova, E.F., Slivkin, A.I. (2013). Determination of biologically active substances in the fruits of sea buckthorn Hippophae rhamnoides L. Chemistry of vegetable raw materials, 3:181-186.
Trineeva, O.V., Slivkin, A.I., Dortgulyev, B. (2015). Investigation of the microelement composition of fruits of sea buckthorn. Bulletin of the Voronezh State University. Series: Chemistry, Biology. Pharmacy, 2:124-128.
Upadhyay, N.K., Kumar, M.Y., Gupta, A. (2010). Antioxidant, cytoprotective and antibacterial effects of Sea buckthorn (Hippophae rhamnoides L.) leaves. Food and Chemical Toxicology, 48:3443-3448.
Pokharel, Y.R., Kunwar, R.M., Bussmann, R.W., Paniagua-Zambrana, N.Y., Abbasi, A.M. (2021). Hippophae rhamnoides L. ssp. turkestanica Rousi Hippophae rhamnoides L. Hippophae salicifolia D. Don Hippophae tibetana Schltdl. Elaeagnaceae. In Ethnobotany of the Himalayas. Cham: Springer International Publishing, pp:1033-1041.
Yamansarova, E.T., Kukovinets, O.S., Salimova, E.V., Plakushkina, D.Yu., Abdullin, M.I. (2008). Composition of neutral lipids of sea buckthorn pulp oil Hippophae rhamnoides L. Bulletin of the Bashkir University, 13:18-19.
Zaitsev, G.N. (1973). Methods of biometric calculations. M.: Nauka.
Zeb, A. (2006). Anticarcinogenic potential of lipids from Hippophae; Evidence from the recent literature. Asian Pacific Journal of Cancer Prevention, 7:32.
Zhengyi, W., Raven, P.H., Hong, D.Y. (2007). Flora of China (Clusiaceae through Araliaceae).
Author Info
G.S. Аidarkhanova1*, S.A. Kubentayev2, K.S. Izbastina1,2 and N.A. Bizhanova32Astana Botanical Garden Branch of the Institute of Botany and Phytointroduction, Kazakhstan
3Department of Biodiversity and Bioresources, Faculty of Biology and Biotechnology, Al-Farabi Kazakh National University, Kazakhstan
Citation: Аidarkhanova, G.S., Kubentayev, S.A., Izbastina, K.S., Bizhanova, N.A. (2022). Population status and fruit safety assessment of Elaeagnus rhamnoides (L.) A. Nelson (Elaeagnaceae Lindl.) in Kazakhstan. Ukrainian Journal of Ecology. 12:1-8.
Received: 08-Oct-2022, Manuscript No. UJE-22-76769; , Pre QC No. P-76769; Editor assigned: 10-Oct-2022, Pre QC No. P-76769; Reviewed: 20-Oct-2022, QC No. Q-76769; Revised: 25-Oct-2022, Manuscript No. R-76769; Published: 31-Oct-2022, DOI: 10.15421/2022_403
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
