Original Article - (2022) Volume 12, Issue 2
Quality control of surface water of Mexa dam and its suitability for irrigation (El Tarf region)
A. Bergal1*, W. Boumaraf1, A. Delimi1 and D.E. Benouareth2Abstract
Water is a natural element essential to life; it is a necessary wealth for any human activity. With the aim of seeing the aptitude of the water of Mexa dam North East Algerian, we carried out the present study. In order to evaluate its aptitude to ensure certain natural functions and the supply of drinking water, irrigation and industry, we carried out a physico-chemical and bacteriological analysis of the waters of the basin the year 2020.
The purpose of this study was to evaluate the quality of the water in the Mexa dam. Three sampling stations were selected over two months to determine the physicochemical and bacteriological parameters of the water. Some physicochemical parameters and heavy metal concentrations were analyzed in water samples (T, pH, EC, TDS, DO, Salinity, Turbidity, durability, Cl–, Ca2+, Mg2+, NO2, NO3– and NH4+). The samples taken for the bacteriological study was have focused on the research and enumeration of total germs, faecal coliforms and faecal streptococci.The results indicated that most of the physicochemical parameters of the Mexa dam were within or at the permissible limit of Algerian standards and WHO for drinking and irrigation water. The establishment of the water suitability map of the Mexa dam for irrigation confirms the results obtained. The bacteriological results obtained showed the presence of significant levels of totale and faecal coliforms. The high load of these germs during the study period may be due to runoff and unhygienic behavior observed in the study area.
Keywords
Mexa dam, Water, Physical and chemical quality, Irrigation.
Introduction
Water a main natural resource and valuable national resource, is the main element of the ecosystem. Water sources can be mainly in the form of rivers, lakes, glaciers, rainwater, groundwater, etc., (Guechi, 2016). In addition to the need for water for drinking, water resources play a vital role in various sectors of the economy such as agriculture, livestock, forestry, industrial activity, hydropower generation, fisheries and other creative activities. The availability and quality of water, both surface and groundwater, has been deteriorated due to some important factors such as population increase, industrialization, urbanization, etc., (Guechi, 2016).
To prevent this source, it is necessary to assure a sustainable management of this resource and to preserve the environment in which water is in continuous interaction, the biosphere. Water is essential to life, but it is also responsible for the death of millions of human beings in the Third World because of its pollution by chemicals and microbiological products that make it unfit for consumption (Guiraud, 1998).
Therefore, in recent years the control of pollution and water quality become particularly obligatory explodes. In order to preserve the environment and the health of living beings or to exploit it for human consumption or industrial, use (Barbault, 2007). The Water Quality of a specific area or specific source can be assessed using biological, physical and chemical parameters. The values of these parameters are harmful to human health if they occurred over defined limits (Guechi, 2016). Therefore, the suitability of water sources for human consumption has been described in terms of the water quality index (WQI), which is one of the most effective ways to describe water quality. The phenomenon of water pollution contributes significantly to the limitation of drinking water resources (Aissaoui and Houhamdi, 2008).
The evolution of the physico-chemical parameters of the water influences the characteristics of the soil, which will have repercussions on the agricultural activity yield. The chemical study of irrigation waters is necessary to highlight the danger that some chemical elements present, for the plants that support poorly the soils saturated in sodium and salt [(Lazhr, 2011).
For this reason, this work aims to study the physico-chemical and microbiological quality of Mexa dam water which characterized by an intense anthropic influence manifested by agricultural activities practiced throughout the catchment area and by various activities related to the agglomerations; and to establish a comparison with the World Health Organization standards for drinking water.
Materials and Methods
Description of the study area
Today Algeria has more than 70 dams in service with a total capacity of 7.7 billion m3 and allowing to regulate an annual volume of 3.6 billion m3 used for drinking water supply, industry and irrigation. Among these dams, the Mexa dam (Fig. 1) is located 75 km from the city of El Tarf. The objective of this dam is to supply the cities of Annaba, El Tarf and El Kala with drinking water. It was built in 1994, commissioned in 1998; its total capacity is 47 hm3/year. The average annual input is 103 hm3/year, the surface of the catchment area is 650 km, with a height of 40 m and a length of 402 m, the normal retention level is 52 m, the highest water level is 58.33 with a spillway with a free threshold of 1800 m3 (Direction of the dam, 2020).
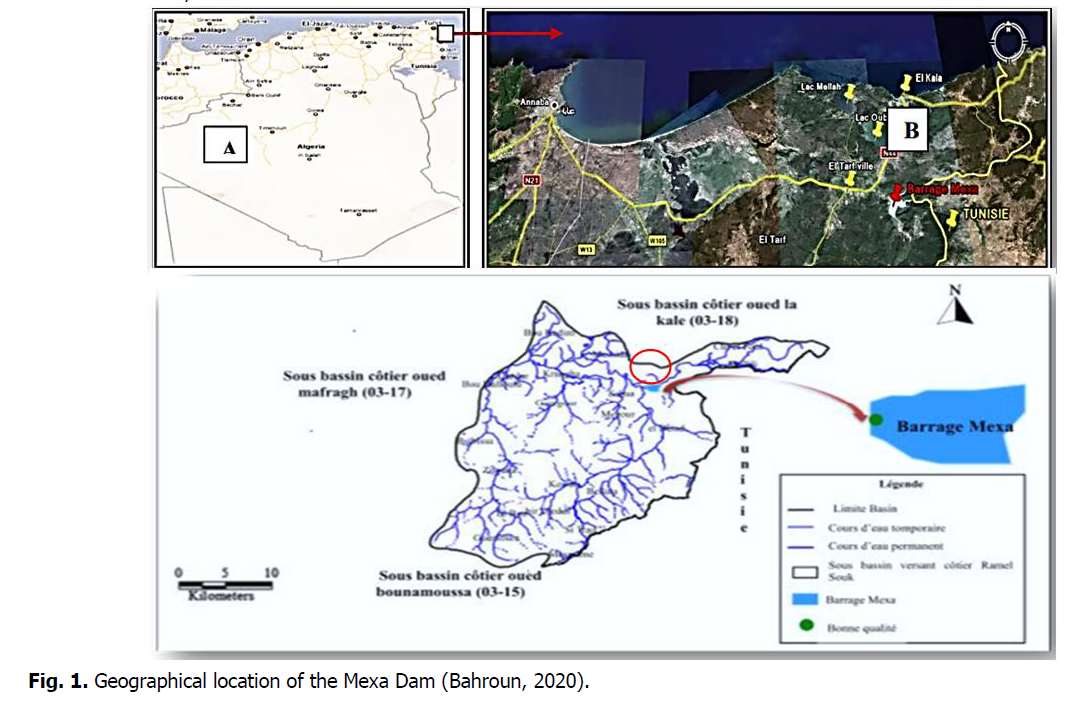
Fig 1. Geographical location of the Mexa Dam (Bahroun, 2020).
The Mexa dam is located in the commune of Bougous willaya of El Tarf, (near the Algerian-Tunisian border), 8 km from the capital of the wilaya El Tarf, and 71 km from the city of Annaba following the national road N°44. The Mexa dam is located at the level of the coastal sub-basin Ramel Souk 03-16, it controls a catchment area of 579 Km², It is limited (Fig. 1):
• To the North by the coastal sub-basin oued oued la Kale 03-18;
• To the West by the coastal sub-basin oued Mafragh 03-17;
• To the South by the coastal sub-basin oued Bounamoussa 03-15;
• To the East by the Tunisian border.
The dam has a double role:
• The supply of drinking water to the city of Annaba and the following centers in the willaya of El Tarf:El Tarf city, El Kala, Souarahekh, El Aioun, Ramel Souk, Dréan, Besbes, Ben M'hidi, El Chatt, Ben Amar, Ain Assel.
• Regulation of the floods of the Oued Kebir and reduction of the floods towards the plains of El Tarf.
Sampling and choice of sampling sites
The objective of our work is to evaluate the quality of Mexa dam water intended for irrigation, based on a follow-up analysis of physicochemical and bacteriological parameters. This study will allow us to determine the suitability of Mexa dam water during this study period for irrigation.
The sampling was carried out in situ, in winter period of 2020 extended over two months at a rate of three sampling per month. For surface water (superficial water), the sterile bottles are plunged at a distance that varies from 20 to 30 cm from the surface far from the edges, as well as from natural or artificial obstacles (Rodier, et al, 1996). The samples were taken in sterile glass bottles for bacteriological analyses and polyethylene bottles for physico-chemical analyses rinsed at least three times with water to be collected. After bottling and labeling, the sample should be placed in a cooler at 4°C to keep it cool.
Physico-chemical analysis of water
Physico-chemical parameters such as temperature (T°), hydrogen potential (pH), electrical conductivity (EC), salinity, total dissolved solids (TDS), durability and turbidity, were measured in situ using a multi-parameter field instrument (type Hanna Hi 8519N). The monitoring of physico-chemical parameters is carried out in the laboratory according to the technique of these parameters are: Total alkalimetric titre (TAC), calcium (Ca+2), magnesium (Mg+2), sodium (Na+), chloride (Cl-), ammonium (NH4+), nitrate (NO3-), nitrite (NO2-) (Table 1).
| Parameters | Instrument/Method of analysis | Unitis WHO standards 2011 |
|---|---|---|
| Temperature | Thermometer | C°25 |
| pH | pH meter | pH units 6,5 à 8,5 |
| EC | Conductivity meter | µS/cm 1500 |
| Turbidity | Turbidity meter | NTU 5 |
| DO | Oxymeter | %O2 80 |
| Salinity | Conductivity meter | mg/l <160 |
| Cl-, Ca++, Mg++ | Titrimetric method | mg/l 250-200-12 |
| NO3-, NO2-, NH4+ | Spectrophotometer | mg/l 3-50-0.5 |
| TDS | TDS-meter | mg/l 600-1000 |
| Hardness/Durability | Titrimetric method mg/l | 200 |
| TA | Titimetry F° | 100 |
Table 1. Different instruments or methods and WHO standards for parameters analysis.
Bacteriological study
The study of bacteriological parameters was determined by the Millipore membrane filtration method (0.45 μm) and concerned on the quantification of faecal origin parameters:faecal coliforms (FC) and total coliforms (TC). This method utilizes elevated temperature incubation to distinguish faecal coliforms from the total coliforms. For faecal streptococci group, the counts were made with filter membrane on Slanetz and Bartley Agar. The Results are expressed as colony forming units per unit of volume.
Results and Discussion
Physico-chemical characteristics of the water
The physico-chemical characteristics give an idea about the water quality in any water body.
Temperature (Tº)
The temperature of surface waters depends on the thermal exchanges with the ambient air and the solar radiation. The temperature influences parameters such as oxygenation, conductivity, solubility of different substances, etc. It also plays an important role in the increase of chemical and bacterial activity and water evaporation. It varies according to the external air temperature (season), the geological nature and the depth of the water. Any sudden variation of this parameter leads to a disturbance in the balance of the aquatic ecosystem (Chapman and Kimstach, 1996).
Fig. 2 shows water temperature values that are higher than 25°C. The latter is considered an indicative limit value set by the World Health Organization (WHO) for water intended for crop irrigation. This increase in temperature is due to the climate change that particularly affects the region. The temperature of the water in the dam varies according to the outside temperature (the area), so their variation is seasonal, the highest values are recorded during the summer season (Max value is 28) and the lowest during the winter season (Min value 12).
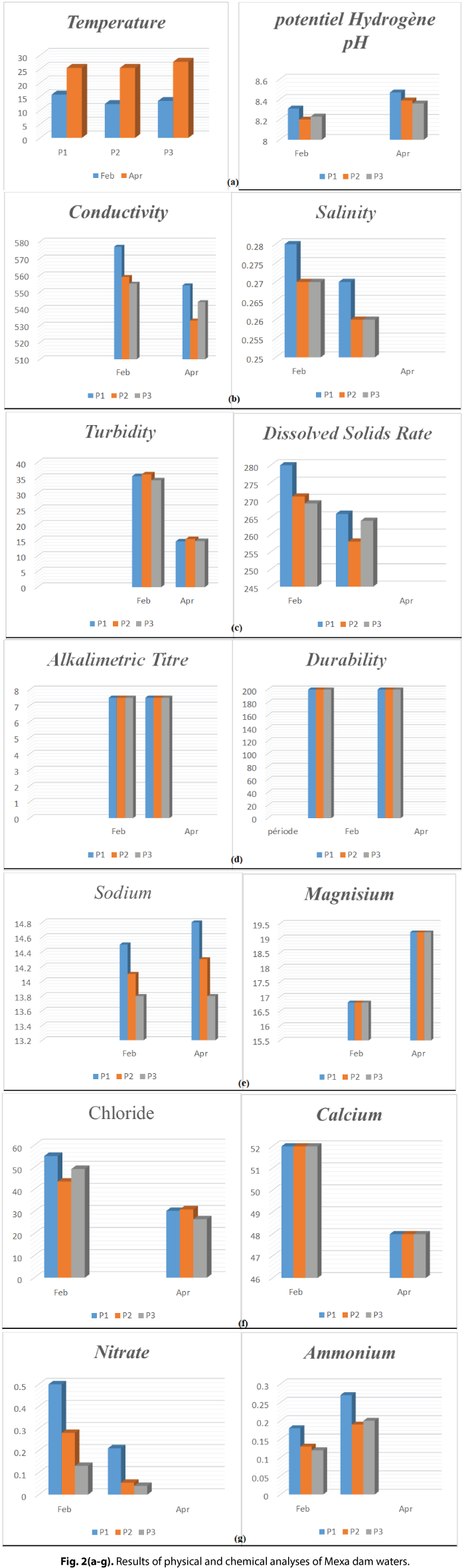
Fig. 2(a-g). Results of physical and chemical analyses of Mexa dam waters.
Potential of hydrogen (pH)
The pH (Hydrogen Potential) measures the concentration of H+ ions in water, and thus reflects the balance between acid and base on a scale of 0 to 14. This parameter characterizes a large number of physico-chemical equilibrium and depends on multiple factors, including the origin of the water. The pH of natural waters is related to the nature of the soil through which it flows. The pH influences most of the chemical and biological mechanisms in water. Usually, the pH values are between 6 and 8.5 in natural waters (Chapman and Kimstach, 1996). It decreases in the presence of high levels of organic matter and increases during low water periods, when evaporation is important (Meybeck, et al, 1996).
Raw water from Mexa Dam during the study period has a pH that does not exceed potability standards (6.50<PH<8.50). These results suggest that water flows through limestone-rich soils at the Mexa watershed. Our pH levels are almost similar to those obtained in Bounamoussa River which varied between 6.94 and 8.02 (Ramdani and Laifa, 2017).
Electrical Conductivity (EC)
Conductivity measures the capacity of water to transmit an electric current. It is directly proportional to the quantity of salts (ions) dissolved in the water. Conductivity is also a function of water temperature, it is more important when the temperature increases. It is also used to assess the amount of salts dissolved in the water (Rodier, 1984).
The conductivity of the water in the dam fluctuates between (533 μS/cm April and 559 μS/cm Feb) and it is noted that it does not exceed the WHO standards. Usually the variation of the conductivity is in function of the precipitations, the low values are marked during the rainiest months because of the dilution, while those of the least rainy months are the most conductive of electricity because of the concentration of minerals in the waters after the season of evaporation. This observation allowed us to appreciate the mineralization and other chemical reactions related to it (Guechi, 2016). We can deduce that these waters vary from a water of mineralization which can accentuated to a water of average mineralization.
Salinity
Salinity is an ecological factor specific to aquatic biotopes (but also to soils) which characterize their salt content (NaCl) and other salts dissolved in water. The presence of salt in water modifies some properties (density, compressibility, freezing point, temperature of maximum density), others (viscosity, light absorption) are not significantly influenced. Finally, some properties are essentially determined by the quality of the salt in the water (conductivity, osmotic pressure) (Merzoug, 2009).
The results obtained from the study field show that the salinity of the waters varies between 0.26 and 0.28. The latter represents lower values compared to the work of Sekiou and Kellil (2014).
Turbidity
Turbidity is an organoleptic parameter (Ghazali and Zaid, 2013). The latter is due to the presence of particles in suspension, especially colloidal; clays, silts, grains of silica, organic matter, etc., which give a cloudy appearance to the water (Graindorge, 2015). The assessment of the abundance of these particles measures its degree of turbidity (Rodier, et al., 2009). High turbidity can allow microorganisms to adhere to suspended particles and reduce the light that aquatic plants use for photosynthesis. Turbidity is expressed in turbidity units (NTU) and should be less than 5NTU (Bengoumi, et al., 2013).
These data allowed us to observe the increase of the turbidity from where the report of the presence of the clayey particles in suspension in water and in addition it is added by the passage of wild animals which drink from where the disturbance of the water of the source.
Dissolved Oxygen (DO)
Oxygen is one of the particularly useful parameters for water and is an excellent indicator of its quality. It is one of the most sensitive parameters to pollution (Makhoukh, et al., 2011).
Raw water from Mexa Dam during the study period has dissolved oxygen that exceeds potability standards (20 mg/l).
Dissolved Solids Rate (TDS)
Dissolved solids consist mainly of inorganic substances dissolved in water. The main constituents of dissolved solids are chlorides, sulfates, bicarbonates, calcium, magnesium and sodium. They come from natural sources, industrial sources, soil erosion and atmospheric particulate matter. The most important influence of dissolved solids on water quality is the alteration of taste. They cause sometimes a scaling of the pipes (Makhoukh, et al., 2011).
Durability
The hardness or hydrometric titre of a water corresponds to the sum of the concentrations of metallic cations except those of alkaline metals and hydrogen ion. In most cases, hardness is mainly due to calcium and magnesium ions, to which iron, aluminium, manganese and strontium ions are sometimes added. Hardness is also called calcium and magnesium hardness or soap consumption. It is expressed in milli-equivalents of CaCO3 (Rodier, 2009). The values observed in this study are not variable, the latter would be related to the lithological nature of the formations crossed and in particular to its composition in magnesium and calcium.
Alkalimetric Titre (AT)
The alkalimetric titre allows to appreciate the concentration of all carbonates and bicarbonates in water. The formation of a carbonate layer to protect the pipes against certain risks of corrosion requires a minimum alkalinity. The complete alkalimetric titre, giving the total alkalinity of the water (not only the alkalinity due to the bicarbonate and the carbonate), must not be less than 50°F (Benslimane, 2015).
The values observed in this study are not variable.
Calcium (Ca2+)
(Ca++ ions) is mainly linked to two natural origins:either the dissolution of carbonate formations (CaCO3) or the dissolution of gypsiferous formations (CaSO4).
Fig. 2 allowed us to say that the range of variation of the calcium contents of the dam water is very limited, this variation is mainly related to two natural origins:either the dissolution of carbonate formations (CaCO3), or the dissolution of gypsiferous formations (CaSO4). The range of variation of the calcium content of the water of the dam is very limited, it fluctuates between 52 and 48mg/l. The waters with high Ca contents are those of February 52mg/l always lower than the norms.
Magnesium (Mg2+)
Its origins are comparable to that of calcium, this element comes from the dissolution of carbonate formations with high magnesium content (magnesite and dolomite), its geological abundance (2.1% of the earth's crust) and its high solubility mean that the content in water can be significant (Makhoukh, et al., 2011).
Magnesium is the least abundant major element in the water of the dam than the other elements (cations and anions), the contents in Mg2+ are lower than 30mg/l and thus do not exceed the standards, they vary between 16.8 and 19.2mg/l.
Chlorures (cl⁻)
The origin of chlorides is mainly related to the dissolution of salt formations and the effect of marine salinity. They also come from anthropic pollution and intense evaporation that causes the increase of Cl- ions concentrations, and that occurs in areas where the static level is close to the surface of the ground. The maximum chloride concentration allowed is 250 mg/l, because at higher concentrations the water can taste like salt. Chlorides are also corrosive agents at high concentrations.
Fig. 2 shows that the chloride content of the water of this dam is extremely varied and mainly related to the nature of the land crossed, the origin of chlorides may be due to the passage of water through percolations of sedimentary rocks. The concentrations of Clobs observed at the level of the dam waters show contents lower than the standards of potability 250 mg/l, figure shows that the variation of chlorides is very limited and weak from 26.5 mg/l to 55.2 mg/l.
Sodium (Na⁺)
Sodium Na+ is very abundant on earth. It is found in crystalline rocks and sedimentary rocks (sands, clays, evaporites).
The Fig. 2 allowed us to deduce that the variation of sodium in the water of this dam is very weak and the averages of the seasons do not exceed the norm which is 150 mg/l, the sodium comes partly from the clayey grounds or from the agricultural discharges. Sodium levels allowed by the WHO are around 200 mg/l. The values of sodium oscillate between the minimum value 13.8 mg/l in February and the maximum value 14.8 mg/l in April. According to Guechi (2016), we can see that the highest levels are recorded during the period of low water while the lowest levels are recorded during the period of high water. These data are consistent with our results. These data do not agree with our results.
Nitrates (NO3-)
Nitrates (NO3-) are mineral nutrient ions soluble in water, they are found in the surface layers of the soil, the migration of nitrates increases rapidly on cultivable surfaces left bare exposed during winter (Benslimane, 2015). Nitrates enter the soil and groundwater and flow into rivers (Abboudi, et al., 2014). However, they are also synthetically supplied by fertilizers (Chapman and Kimstach, 1996).
Nitrates are unstable or biochemical plan, between vial filling and laboratory analysis, there is significant biological degradation during transport. In the presence of high concentrations of ammonium, nitrite can also occur through nitrification. For this reason, the samples must be refrigerated. Nitrates are used as an indicator of pollution. They play the role of fertilizer for the plants which assimilate nitrogen in the form NO3.
Nitrate levels are between 0.04 and 0.5 mg/l, while the annual average fluctuates around 2.34 mg/l according to memory. The density of fauna and flora in the watershed of the dam and the organic matter buried in the soil are the origin of nitrates in the water of the dam after a bacterial transformation called "mineralization". In addition to the discharges of communities upstream of the basin, which can also contribute to the significant enrichment of nitrates in the water.
Ammonium (NH4+)
Ammoniacal nitrogen is one of the links in the complex cycle of nitrogen in its primitive state. It is a water-soluble gas. It exists in small proportions, less than 0.1 mg/l of ammoniacal nitrogen in natural waters. Ammonium concentrations provide information on water pollution due to domestic wastewater discharge or to erosion and leaching of agricultural soils (Graindorge, 2015).
Ammonium concentrations provide information on water pollution; as a result of domestic wastewater discharge or erosion and leaching from agricultural soils. Ammonium is not very stable or chemically stable between sampling and laboratory analysis, and there is a significant biological degradation during transport, which is particularly sensitive for low concentrations. Appropriate refrigeration of the sample must be ensured during sampling and transport. The ammonium content of the dam water is highly variable, fluctuating between 0.12 and 0.27 mg/l.
Water suitability for irrigation
Our study region is an agricultural area, an activity that requires a lot of water to meet the needs of various crops. The water used for irrigation must have physical and chemical characteristics that can be tolerated by plants (Graindorge, 2015).
The most used classification of irrigation waters is the one of the American salinity laboratory (USDA) developed by Richards in 1954, based on the combination of the SAR "Sodium Absorption Ratio" with the electrical conductivity in the form of a class diagram (Wilcox diagram). The SAR is given by the formula;
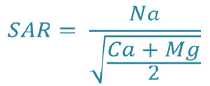
After plotting the two water stations on the Wilcox diagram (Fig. 3 and 4), according to the electrical conductivity and SAR value, the waters of two sources (Maxa dam) belong to the C2S1 class:characterize waters of good quality and can be used without special.
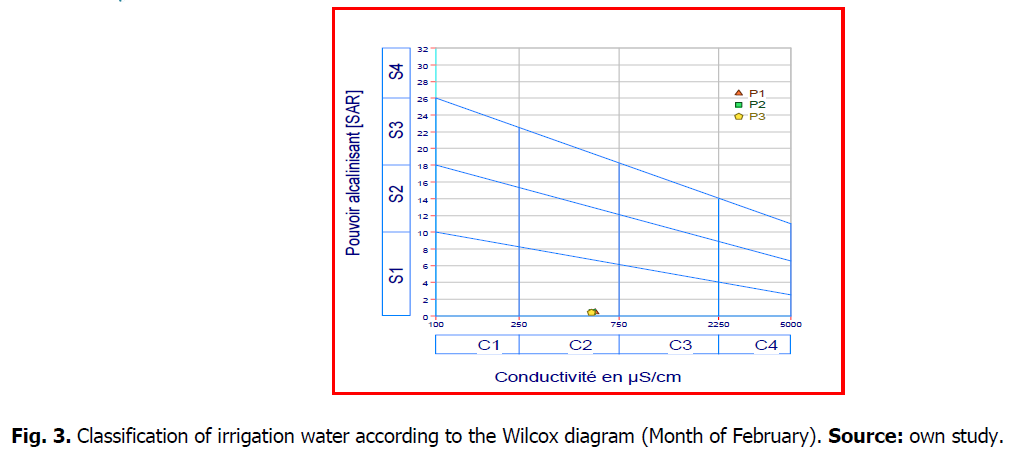
Fig 3. Classification of irrigation water according to the Wilcox diagram (Month of February). Source: own study.
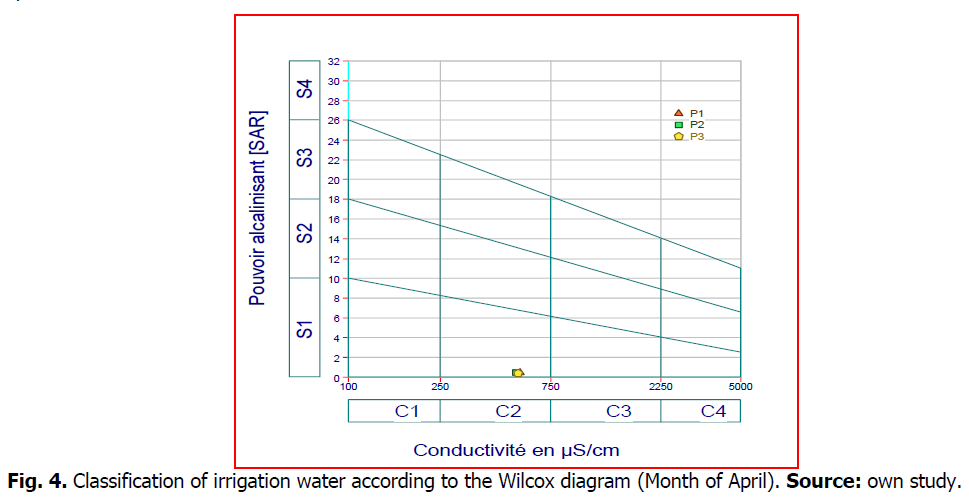
Fig 4. Classification of irrigation water according to the Wilcox diagram (Month of April). Source: own study.
Salinity risk
Salinity has affected agriculture for a very long time. Salts have been recognized as a problem for thousands of years. Mineral salts in water have effects on soil and plants (Chaoui, 2007). Salinity can cause consequential adverse effects due to the binding of sodium from sodium chloride salts by soil colloids. Sodium then exerts a harmful action on vegetation, in an indirect way, by degrading the physical properties of the soil. Under this action, soils become compact and asphyxiating for plants (Todd, 1980).
Richards in 1969 established a scale of quality of irrigation waters according to their salinity evaluated by their electrical conductivity (Table 2).
| Water conductivity | Estimated corresponding salts | |
|---|---|---|
| Excellent | <0.25 | <160 |
| Low salinity | 0.25-0.75 | 160-500 |
| High salinity | 0.75-2.25 | 500-1500 |
| Very high salinity | 2.25-5 | 1500-3600 |
Table 2. Quality of irrigation waters according to their salinity.
Bacteriological analysis of dam waters
Total coliforms
Based on the analysis of the data and the interpretation of the percentage of total coliforms, fecal coliforms and streptococci; our results show values during the three months of study (February and April) as follows (Fig. 5). The majority of the waters are dominated by total coliforms during the month of April.
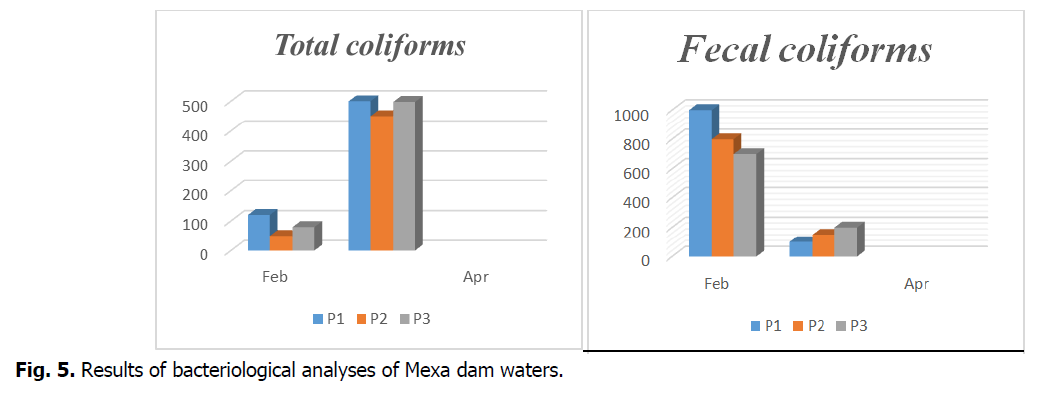
Fig 5. Results of bacteriological analyses of Mexa dam waters.
The results of bacteriological analysis of water show that all samples collected during the month of April were polluted by the presence of coliforms. These are indicators of faecal contamination of the water. The contamination of these waters by total germs could be due to the poor protection of the dam, the ignorance of the elementary rules of hygiene.
Fecal coliforms
Fecal coliforms are very abundant in human and animal intestinal flora, and it is also the only one that is strictly of fecal origin. Fecal coliforms are considered the best indicator of fecal contamination, their presence in water means that the latter is contaminated by pollution of fecal origin and that it may therefore contain pathogenic microorganisms. The source of contamination is near the water resource.
The rate of faecal coliforms are higher than the WHO standards (month of February) (Fig. 4).
The high presence of faecal coliforms in the dam water presents a potential risk for health and human consumption. The discharge of wastewater from several residential units into the dam may also be an important source of streptococci in the water that participate in the contamination of this site. However, a strong faecal contamination in low water period where bacterial multiplication is more favored by temperature, basic pH and alkaline environment, which was supported by other, works (Aboulkacem, et al., 2007; El-Addouli, et al., 2011; Lamrani, et al., 2011). The high concentrations of total coliforms, faecal coliforms and faecal streptococci suggest the possible existence of the wastewater discharge at this dam.
Conclusion
Dams and water reservoirs play an essential role in the framework of water management and contribute to the quality of life of the society. The present work was devoted to the study of the physico-chemical and bacteriological quality of the water of Mexa dam. The study area is subject to a Mediterranean climate, it is characterized by two distinct seasons, one wet rainy with relatively high rainfall and low temperatures, the other dry less rainy with high temperatures.
The raw water from the dam is of good quality, almost all the parameters analyzed are within the standards of potability. For irrigation, the water of the study area is of good quality and does not present any risk for irrigation in the field. The faecal contamination germs counted in the Mexa dam water are due to domestic wastewater discharges and the use of chemical and natural fertilizers (animal droppings).
The classifications of Richards and Wilcox have made it possible to classify the water of the dam, and to show that the salinity is always average at the level of the Mexa dam, the establishment of the suitability map of the water of the Mexa dam for irrigation confirms the results obtained by the two methods; these waters are of good quality.
In general, the water can be used without particular control for the irrigation of plants moderately tolerant to salts, on soils having a good permeability. In the future, it will be necessary not only to assess the health risks related to the level of contamination of these waters, but also to proceed with their treatment before supplying them to consumers.
References
Guechi, A. (2016). Surface waters, physical and chemical characterization and suitability (Reservoir Lake of the Mexa dam northeast of Algeria). Master memory, University of Annaba.
Guiraud, J.P. (1998). Food microbiology. Microbiological Analysis Techniques, Ed, Dunod.
Barbault, R. (2007). Development and ecological diversity: links and connections in Mollard A., Sauboua E., Hirczak M. Territories and issues of regional development: Quae c/o INRA editions, Versailles.
Aissaoui, A., Houhamdi, M., Samraoui, B. (2009). Eco-ethology of scaup nyroca aythya nyroca in lake tonga (Ramsar Site, El-Kala National Park, North-East of Algeria). European Journal of Scientific Research. 28:47-59.
Lazhr, G. (2011). Control of water pollution by acousto-optic method. Memory Presented at the Institute of Optics and Precision Mechanics For the Obtaining of the Diploma of Magister. Ferhat Abbas-Setif University.
Gheid, S., Bahroun, S., Benabdallah, A., Gheid, A. (2021). Evaluation of mexa dam water quality in el tarf region (Extreme North-East Algeria). Food and Environment Safety-Journal of Faculty of Food Engineering, Ştefan cel Mare University-Suceava.
Rodier, J. (1996). Water analysis: Natural waters, wastewater, seawater. Ed., Dunod, 8th Edition, Paris.
Rodier, J., Beuffr, H., Bournaud, M., Broutin, J.P., Geoffray, C.H., Kovacsik, G., Laport, J., Pattee, E., Plissier, M., Rodi, L., Vial, J. (1984). Analysis of water, natural waters, water, seawater. 7th edition. Ed., Dunod.
Chapman, D., Kimstach, V. (1996). Selection of water quality variables. Water quality assessments: a guide to the use of biota, sediments and water in environment monitoring. Chapman edition, 2nd ed., E & FN Spon, London, pp:59-126.
Meybeck, M., Friedrich, G., Thomas, R., Chapman, D. (1996). Rivers water quality assessments: a guide to the use of biota, sediments and water in environment monitoring, Chapman edition, 2nd ed., E & FN Spon, London, pp:49-58.
Ramdani, H., Laifa, A. (2017). Physicochemical quality of Wadi Bounamoussa surface waters (Northeast of Algeria). Journal of Water and Land Development, 35:185.
Rodier, J., Beuffr, H., Bournaud, M., Broutin, J.P., Geoffray, C., Kovacsik, G., Laport, J., Pattee, E., Plissier, M., Rodi, L., Vial, J. (1984). Analysis of water, natural waters, water, seawater. 7th edition. Ed., Dunod.
Merzoug, D., Khiari, A., Aït Boughrous, A., Boutin, C. (2010). Aquatic fauna and water quality from wells and springs in the region of Oum-El-Bouaghi (North-East of Algeria). Hydroecology Application, 17:77-97.
Sekiou, F., Kellil, A. (2014). Characterization and empirical graphical and multivariate statistical classification of bottled spring waters from Algeria. Hydraulics, 20:1112-3680.
Ghazali, D., Zaid, A. (2013). Study of the physico-chemical and bacteriological quality of the waters of the Ain Salama-Jerri spring (region of Meknes-Morocco). Larhyss Journal, 12:25-36.
Graindorge, J. (2015). Guide to water quality analyzes. Territorial edition, Voiron, Paris, p:126.
Rodier, J., Legube, B., Merlet, N. (2009). The analysis of water, 9th Edition, Ed. Dunod, 1579 p.
Bengoumi, D., Chahlaoui, A., El Moustaine, R., Belghiti, L., Samih, M. (2013). Typology of well water quality used for poultry watering (Gharb and Meknes Morocco), Mersenne editions. Science, Lib, 5:1-23.
Makhoukh, M., Sbaa, M., Berrahou, A., Van, M. (2011). Clooster, Contribution to the physico-chemical study of surface water from the Moulouya wadi (eastern Morocco). Larhyss Journal, pp:149-169.
Benslimane, M., Hamimed, A., Khaldi, A., El Zerey, W. (2015). Methodological approach to the evaluation of the water management policy in wetlands in the case of Chott Chergui (southwest Algeria). LARHYSS Journal.
Abboudi, A., Tabyaoui, H., El Hamichi, F., Benaabidate, L., Lahrach, A. (2014). Study of the physicochemical quality and metallic contamination of surface waters of the Guigou watershed (Morocco). European Scientific Journal.
Chaoui, W. (2007). Impact of organic and chemical pollution of the waters of the Oued Seybouse and the Oued Mellah on the groundwater of the alluvial aquifer of boucegouf (Guelma), master's thesis, Faculty of Earth Sciences, University of Annaba, pp:79-84.
Todd, K. (1980). Ground water hydrology. John Wiley and Sons, 2nd Edition, New York, USA.
Aboulkacem, A., Cahlaoui, A., Soulaymani, F., Rhazi, F., Benali, D. (2007). Comparative study of the bacteriological quality of the waters of Oueds Boufekrane and Ouislane crossing the city of Meknes (Morocco). Review Microbiology Ind San and Environment, p:10-22.
El-Addouli, J., Lamrani, H., Chahlaoui, A., Ennabili, A. (2011). Assessment of the physicochemical and bacteriological quality of the Boufekran wadi in the vicinity of the effluents of the city of Meknes (Morocco). Science Lib Editions Mersenne.
Lamrani, H., Chahlaoui, A., El addaouli, J., Ennabili, A. (2011). Assessment of the physicochemical and bacteriological quality of the Boufekrane Oued in the vicinity of the effluents of the city of Meknes (Morocco). Science Lib.
Author Info
A. Bergal1*, W. Boumaraf1, A. Delimi1 and D.E. Benouareth22Faculty of Natural and Life Sciences and Earth and Universe Sciences, University 8 Mai 1945 Guelma, BP 401, 24000, Guelma, Algeria
Citation: Bergal, A., Boumaraf, W., Delimi, A., Benouareth, D.E. (2022). Quality control of surface water of Mexa dam and its suitability for irrigation (El Tarf region). Ukrainian Journal of Ecology. 12:26-35.
Received: 24-Jan-2022, Manuscript No. UJE-22-52845; Accepted: 21-Feb-2022, Pre QC No. P-52845; Editor assigned: 28-Jan-2022, Pre QC No. P-52845; Reviewed: 08-Feb-2022, QC No. Q-52845; Revised: 14-Feb-2022, Manuscript No. R-52845; Published: 28-Feb-2022, DOI: 10.15421/2022_341
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
