Research Article - (2021) Volume 11, Issue 2
Abstract
We studied the changes in morphophysiological (gonadosomatic index, hepatosomatic index, spleen index) and biochemical (protein, glycogen, lipids, malonic dialdehyde, thyroxine, triiodothyronine, and cortisol) parameters in redfish under the action of toxic loading. Two lakes were chosen as model zones. These reservoirs differ significantly in anthropogenic impact and water quality (oxygen content and mineralization). The high content of toxicants (heavy metals) in Lake Kyrylivske significantly affected the physiological status of the studied fish. In the case of intensive toxic pollution of the reservoir, the indicators of the liver and spleen in redfish increase by 1.9 and 1.6 times, respectively that testified to the increasing functional activity of these organs. Changes in energy substrates accompanied enhancement of the functional activity of internal organs. The protein and glycogen content in the tissues of the liverwort from the contaminated lake was lower by 24.5% and 36.0%, respectively, compared with the conditional control. We found that the anthropogenic load on the reservoir protein content in the gills of fish increases by 18.2% (p≥0.05). However, under these conditions, its content in the muscles is significantly reduced by 24.5% compared to fish from relatively clean water. Confirmation of chronic stress at the time of the study was the high value of malonic dialdehyde in liver tissue. In fish from Lake Kyrylivske (dirty reservoir), its content was higher by 40.7% (p ≥ 0.05) than the same indicator for conditional control. Significant changes in hormone content also occurred during the stay of the Rudd in unstable hydro- ecological conditions. Thus, we found that the content of triiodothyronine in the blood plasma of fish under the influence of toxic contamination was 2.9 times lower (p≤0.05) compared to fish from control conditions. However, the content of thyroxine in the blood plasma of redfish from the contaminated lake was higher by 40.0% (p≤0.05) relative to the conditional control. The content of thyroxine and triiodothyronine in the blood plasma allows assessing the strength of anthropogenic impact on fish adequately. The obtained data on the physiological state of the Rudd allows monitoring (by changes in internal organs, by the content of hormones, malonic dialdehyde) the ecological state of water bodies located in geographically urbanized areas. This will further help minimize the harmful effects of toxic loads on the aquatic ecosystems of the city and its environs.
Keywords
toxic pollution, adaptive reactions, morpho-physiological parameters, hormone content, biomonitoring
Introduction
Pollution of aquatic ecosystems is attracting the attention of researchers around the world. High levels of urbanization are developing rapidly around the world, leading to the increased environmental impact of cities (Romanenko et al., 2015; Kalyn et al., 2020; Bashchenko et al., 2020; Borshch et al., 2020; 2021; Honcharova et al., 2021; Vasylyev et al., 2021; Butsiak et al., 2021; Saranchuk et al., 2021; Kalaitan et al., 2021). The consequence is the pollution of urban water bodies. The current state of such reservoirs in the city is of great concern. Given that Kyiv (Ukraine) is a metropolis, the effects of urbanization may have an irreversible impact on the ecological status of water bodies, in particular lakes (Panasyuk et al., 2015). Some studies have shown that the degradation of aquatic ecosystems is caused by excessive dumping of industrial waste, leading to a reduction in the number of commercially important species, leading to economic and social problems. This significantly changes the living conditions for all aquatic organisms. The short-cycle or low-value fish species are the dominant species in fish communities. Groups of fish resistant to stress factors are beginning to predominate in the reservoirs: crucian carp, Chinese, Amur chub (Yevtushenko et al., 2007; Prysiazhniuk et al., 2019; Rudenko et al., 2019; Kofonov et al., 2020). Groups of vulnerable species are depressed, which is reflected in their physiological state. This affects their populations' qualitative and quantitative composition (chub, ruff, fir, and pike perch), reflected in the absence of these species in many excessively polluted water bodies. This poses a threat to the biodiversity of fish communities, in particular, valuable industrial species that can further affect not only the number of individual species, but also determine the overall fish productivity of reservoirs.
Various methods and strategies for monitoring natural water bodies and watercourses are used to assess environmental pollution (Kumar et al., 2004; Fernandes et al., 2008). It is known that the methods of biochemical indicators of the ecological status of water bodies are susceptible. A living organism can respond to lower concentrations of toxic substances than any analytical sensor (Rudneva, 2006). By studying these variations, it is possible to identify the degree of stress and predict the possible detrimental effects on individual members of fish communities.
Among aquatic animals, one of the ultimate "targets" of toxic pollution is fish (Viana & Lucena Fredon, 2009). Fish is widely used to assess the quality of the aquatic environment and, as such, can serve as a bioindicator of environmental pollution. The use of biochemical approaches in water monitoring and early detection of pollutants is becoming increasingly popular (de La Torre et al., 2005; Viarengo et al., 2007). Biological monitoring is a method of assessing water quality through the response of biological groups to changes in environmental conditions.
Therefore, today one of the most critical tasks is to discover and implement inexpensive and effective methods of environmental indicators. The question of the choice of indicators for bioindication remains open. An important role is given to the choice of model objects for biomonitoring. It is advisable to use those species of fish that occupy different ecological niches, taxonomically separated from each other and have wide limits of distribution (Prychepa et al., 2017). One such species may be the Rudd, which is an essential component of the fish communities of most water bodies in Eastern and Central Europe, including Ukraine. It is an excellent biological model for toxicological research. This is evidenced by a variety of species characteristics, namely its effectiveness in adapting to a variety of diets, high resistance to disease, rapid reproduction. Rudd is one of the main freshwater fish, widespread in Europe and Central Asia, in the basins of the North, Baltic, Black, Caspian and Aral Seas. It was artificially imported to Ireland, the United States, Morocco, Madagascar, Norway, Tunisia, New Zealand, Canada and Spain. Rudd is a pelagic species that inhabits areas of water bodies with significant development of higher aquatic plants (macrophytes) (Guinan. This species is a convenient object for comprehensive ecotoxicological assessment of the aquatic environment, including metropolitan areas. This is facilitated by the peculiarities of the physiology of this species, in particular its behavioral responses, non-standard food choices, resistance to oxygen, salt and gas conditions project for monitoring various reservoirs, including those heavily contaminated with petroleum products.
This work aimed to study the change in the biochemical parameters of Rudd in response to excessive anthropogenic impact with the subsequent possible use of this species for biomonitoring of the aquatic environment in geographically urbanized areas.
Materials and Methods
Research area. The research was conducted on two lakes in Kyiv (Ukraine), which are characterized by different hydrochemical and toxicological parameters. Reservoir № 1 (Lake Kyrylivske) was characterized by a significant anthropogenic load. The lake is located at the geographical coordinates of 50º29'52''N 30º29'38" E. The lake's average depth was 12 m, maximum 15 m; the area was 19.5 hectares. The lake is used as a collector of wastewater coming from car washes, gas stations, industrial shops, road washes.
Concentrations of major toxicants and hydrochemical parameters of lake water are given in Table 1. Reservoir N 2 (Lake Babyne) is characterized by a small anthropogenic load. The reservoir is located at coordinates 50º28N 30º32'42''E. The lake is located on an island in the middle of the Dnipro River and is separated from the city's urban areas. The lake's water surface area is 4.72 ha, the average depth is 2 m, and the maximum is 5 m. The specified water body is taken for conditional control (Fig. 1).
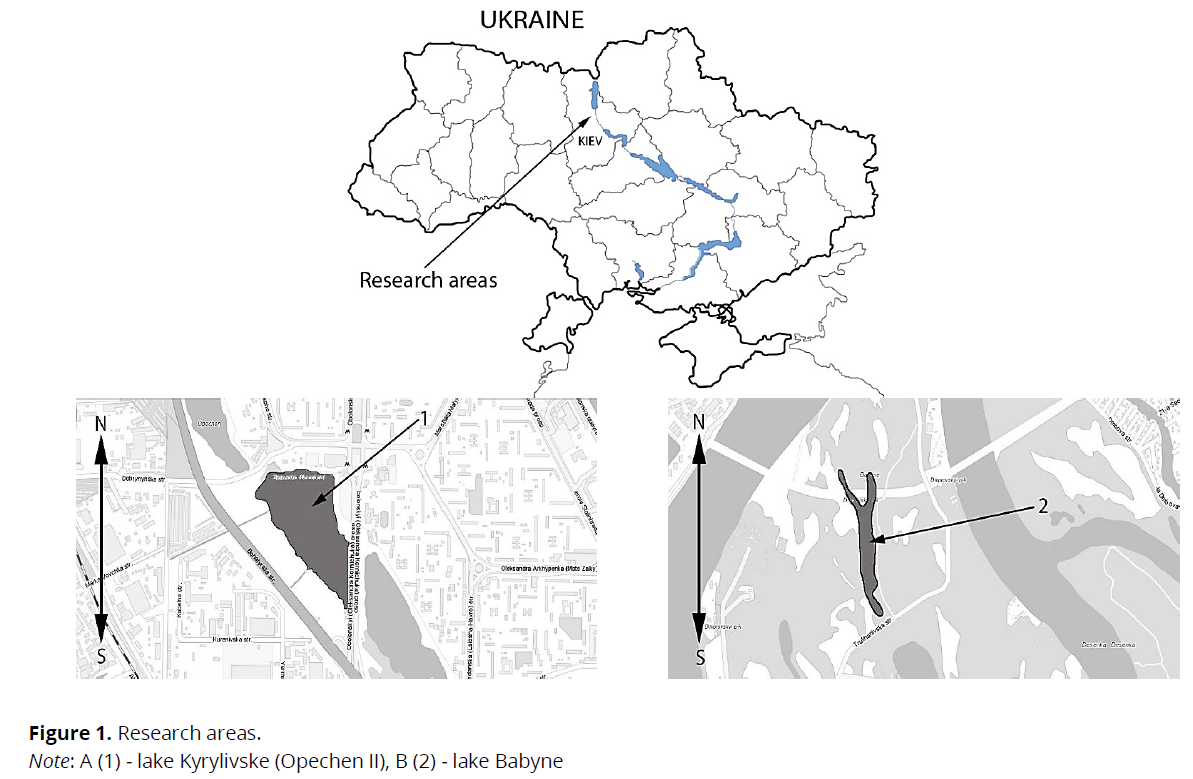
Fig 1: Research areas.
Note: A (1) - lake Kyrylivske (Opechen II), B (2) - lake Babyne
Determination of hydrochemical, hydrophysical and toxicological parameters. Winkler's method determined the content of dissolved oxygen in the water (Romanenko, 2006). the pH of the water was evaluated using the pH meter PH-009. The total water salinity was determined using an IDS-2 TDS meter. The water temperature was measured using a mercury thermometer. The content of metals (Zn, Cd, Co, Ni, Fe, Mn, Mg, Ca, Na, K, Cu) in the studied lakes were measured after concentrating the water samples by boiling on a C-115 atomic absorption spectrophotometer using the appropriate standards (15) and evaluated in μg/l (Novikov et al., 1990).
Determination of morpho-physiological parameters. The object of study were experimental groups of fish aged 3 years. 10- 15 individuals were used for morpho-physiological analysis. Total (TL), standard (SL) length, fish weight (P), catfish, gonads, liver and spleen were measured. The Clark fattening factor (CF) was calculated by the formula CF = P (g) × 100 / SL3 (cm) (Pravdin, 1966). Gonadosomatic index (GSI), hepatosomatic index (HSI) and spleen index (SI) were calculated using the weight of fish from the equations: GSI% = (gonad weight (g) / fish weight (g) × 100; HIS% = (liver weight (d) / weight of fish (g) × 100; SI% = (mass of spleen (g) / weight of fish (g) × 100) (Pravdin, 1966).
Biochemical parameters. 6 individuals of Rudd were used for biochemical analysis. The glycogen content (mg/g) was determined using an anthrone reagent according to the described procedure (Severin & Solov'yev, 1989). The Lowry method determined the total protein content in muscle tissue, gills, and liver (mg/g) (Lowry et al., 1951). The content of total lipids was evaluated using a phosphorus reagent using standard commercial kits "Total lipids" ("Phyllis-Diagnosis"). These parameters were determined photocolorimetrically using 2 MP KFK. The level of lipid peroxidation was determined by standard methods (Stalnaya & Garishvili, 1991) based on the reaction of malonic dialdehyde with thiobarbituric acid, forming a colored trimethine complex. Blood from fish was taken from the heart using a heparinized syringe. Plasma was obtained after centrifugation of whole blood for 15 minutes at 6000 rpm. The total content of thyroxine (T4), triiodothyronine (T3), and cortisol in fish plasma was determined by enzyme-linked immunosorbent assay using commercial T3-IFA, T4-IFA (Granum Research and Production Laboratory, Ukraine) and DS-IFA-steroid cortisol NVO "Diagnostic Systems", Russia) with Rayto RT-2100C IFA analyzer.
Statistical methods. Statistical data processing was performed by conventional methods using software packages for personal computers Statistica 8.0 (StatSoftInc, USA). All results are given as mean ± standard deviation (SD). Their correspondence for the difference between the data samples was determined by one-way analysis of ANOVA variance at a significance level of p<0.05.
Results and Discussion
Table. 1 presents the results of a study of water quality indicators, in particular pH, water salinity, and the content of dissolved oxygen in water. It is established that in the lake Kyrylivske the higher total mineralization of water in comparison with lake Babyne. Similar results have already been observed in previous studies (Romanenko et al., 2015).
| Indicator | Kyrylivske Lake (polluted reservoir) | Lake Babyne (conditional control). |
|---|---|---|
| Dissolved oxygen, mg/L | 6.5–8.9 | 8.0–11.0 |
| pH | 7.8–8.2 | 8.2 |
| Water mineralization, mg/L | 540–620 | 250–281 |
| Sulfates, mg/L | 60–137.0 | 22.2–27.6 |
| Chlorides, mg/L | 118.8–133.5 | 15.51–19.9 |
| Temperature, °C | 22.0–24.6 | 20.0–22.0 |
Table 1. Water quality indicators
Most of the city's water bodies are subject to significant anthropogenic pollution. They contain many heavy metal ions, petroleum products, phenols, and other toxicants (Romanenko et al., 2015, Zhezherya et al., 2016). We focus on indicators (close to natural) and modified (with excessive urbanization) water bodies.
A characteristic feature of the studied lakes is that the Rudd was dominant in the structure of their fish communities (Romanenko et al., 2015). This species has a wide range. Therefore, it was chosen as a model test object that can integrate adverse effects through trophic food connections. It occupies the surface horizon of water, actively changes habitat, and feeds mainly on macrophytes and periphyton. Macrophytes are known to accumulate toxic compounds, including heavy metals (Fedonenko et al., 2008). In addition, due to anthropogenic eutrophication, there is an intensive development of filamentous and blue-green algae in polluted water bodies (Panasyuk et al., 2015). This causes a lack of oxygen in the water and the uncontrolled development of saprophytic bacteria. Chlorides, sulfates, and sodium ions come from wastewater. Due to this, the total mineralization of water increases. In our case, up to 620 mg/L in Lake Kyrylivske compared to Lake Babyne (250–281 mg/L). Table 2 illustrates the difference in the content of some toxicants between the two studied lakes. Thus, the content of Ni, Cu, Co in Kyrylivske was higher than in Lake Babyne (conditional control). According to the literature, the level of oil products in Kyrylivske Lake is higher than in Lake Babyne. The high content of heavy metals was also noted in the paper (Zhezherya et al., 2016).
| Indicator | Kyrylivske Lake (polluted reservoir) | Lake Babyne (conditional control) |
|---|---|---|
| Nickel, µg/L | 52 | 11 |
| Copper, µg/L | 39.4 | 8 |
| Manganese, µg/L | 90.9 | 80.5 |
| Cadmium, µg/L | 0.06 | ≤0.05 |
| Cobalt, µg/L | 2.2 | 0.59 |
| Iron, µg/L | 670 | 540 |
| Petroleum products, mg/L | 0.199–0.492 | 0.085 |
| Sodium, mg/L | 18.2 | 6.4 |
| Calcium, mg/L | 70.1 | 52.1 |
Table 2. Characteristics of individual toxicants in the studied reservoirs
The above characteristics of the hydroecological state of reservoirs significantly affected the morpho-physiological and biochemical parameters of the Rudd. According to the study results, the size and weight of the fish were not significantly different (p≥0.05). However, it is known that with long-term chronic pollution of water bodies, the coefficient of fattening of fish decreases slightly (Liebel et al., 2013). However, significant changes were found in the study of organ indices, particularly the liver, spleen. We found that in fish from the contaminated reservoir, hepatosomatic index and spleen index are higher by 1.9 and 1.6 times, respectively, compared with the control (Table 3). The gonadosomatic index in fish from Lake No. 1 was lower than the control by 64.5%. As a rule, the value of the liver index increases in fish from polluted ponds. According to many scientists, the hepatosomatic index (GI) is an informative bioindicator of the body's response to adverse factors (Hori et al., 2006). The detoxification of toxicants and their accumulation occurs in the liver (Sole et al., 2009). Due to the proliferation of liver cells, the body's protective adaptive-compensatory reaction is enhanced. Adverse living conditions that this species encounters from time to time cause significant changes in the functional activity of this organ, which is further reflected in the content of energy substrates and the activity of some enzymes. Some studies have found that the mass of the spleen increases in fish with intense water pollution (Rudneva, 2006). Besides, in response to stress, the blood supply to this organ increases, and then the excretion of erythrocytes is activated. This reaction is due to the need to provide the body with additional oxygen during stressful behavior (Sergeeva & Lukyanenko, 2008). A similar phenomenon was observed in our studies, which may be related to the toxic load on the reservoir from the surrounding areas (Romanenko et al., 2015).
| Indicator | Kyrylivske Lake (polluted reservoir) | Lake Babyne (conditional control) |
|---|---|---|
| Total weight, g | 15.30±4.70 | 19.82±4.61 |
| Total (zoological) length, cm | 11.31±0.75 | 12.32±0.80 |
| Clark fatness,% | 1.72±0.23 | 1.78±0.07 |
| Hepatosomatic index,% | 2.45±0.68* | 1.24±0.24 |
| Spleen index,% | 0.343±0.080* | 0.218±0.034 |
| Gonadosomatic index,% | 1.028±0.250* | 1.696±0.240 |
Тable 3. Morpho-physiological parameters of Rudd from different reservoirs
According to our observations, the gonadosomatic index (GI) was softer in fish from the contaminated lake. This phenomenon was noted by other authors (Rudneva & Kuzminova, 2011). Under conditions of intensive water pollution, the gonadosomatic index of fish decreases due to a decrease in the size of the eggs, while their absolute fertility increases (Shikhshabekov et al., 2000). This post-adaptation effect in fish with prolonged water pollution aims to maintain the population size (Rudneva, 2006). Changes in the internal organs are directly related to the use and synthesis of energy substrates, which have also been involved in the adaptive processes of Rudd in the presence of unstable environmental conditions.
We found that the anthropogenic load on the reservoir protein content in the gills of fish increases by 18.2% (p≥0.05). However, under these conditions, its content in the muscles is significantly reduced by 24.5% compared to fish from a relatively clean body of water (Table 4). Similar results were obtained in model conditions by other researchers (Hori et al., 2006).
| Tissue | Kyrylivske Lake (polluted reservoir) | Lake Babyne (conditional control) |
|---|---|---|
| Gill | 89.57±6.90 | 75.75±16.30 |
| Muscles | 188.70±10.51 | 235.0±10.0* |
| Liver | 85.60±11.57 | 100.2±11.20 |
Table 4. Protein content in the tissues of red perch from different reservoirs, mg/g, M±m, n=6
We registered that the fish content in the pond, which is contaminated with toxic compounds, the protein content was lower. Taking into account the fact that at the time of research in the lake Kyrylivske we recorded a high content of nickel, copper, it could cause inhibition of protein synthesis. This can also be emphasized by insignificant differences in size and mass characteristics, significantly lower in Rudd from a dirty reservoir (see Table 3). The opposite pattern was observed in gill tissues, where the protein content in fish from Lake Kyrylivske was higher than in Babyne (conditional control). This may indicate different functional activity of tissues when using protein (Cicik & Engin, 2005). Cu, Ni, Fe, and Zn are known to perform essential functions in biological systems, such as metabolic rate, but increasing concentrations of these metals can be toxic to muscles (Vatukueu, 2005). Fish accumulate a small amount of glycogen in the liver during periodic lake pollution. The glycogen content also revealed a significant difference between the studied groups of fish. Thus, fish from the contaminated reservoir had a lower glycogen content of 36.0% than fish from the clean reservoir (Fig. 2). This is probably due to its intensive use in the process of detoxification of tissues from toxic compounds. It is known that fish use this energy substrate from the liver as a "strategic source of energy" (Cicik & Engin, 2005). The described decrease in glycogen levels in the liver is confirmed by reports of previous studies conducted on various aboriginal species (Prychepa et al., 2019). Glycogen is the most labile, energy-intensive compound and is used in the processes of adaptation to chronic water pollution, in particular by heavy metal ions (Vatukuru, 2005). It is chemical stress that causes a rapid decrease in carbohydrate content (Vatukuru, 2005; Hori et al., 2006). Changes in the content of total lipids in the liver, which significantly depend on living conditions, mainly feeding, stress levels, and the stage of development of sexual products, were also considered. However, toxic pollution or temperature changes can make their adjustments. Thus, we found that the lipid content of Rudd from a polluted lake (№ 1) was 16.4% higher than that of fish from a clean lake (№ 2) (Fig. 3). The processes of fat accumulation in the liver tissue can have a protective effect, as they are involved in the elimination and detoxification of toxic compounds. This increases the body's resistance to these compounds (Krishna et al., 1994). Also, an increase in the liver's mass during water pollution was found (see table 3). Lipids are less used in urgent adaptation processes. Their metabolism is closely related to maintaining biological balance, especially during periodic toxic stress (Sergeeva & Lukyanenko, 2008).
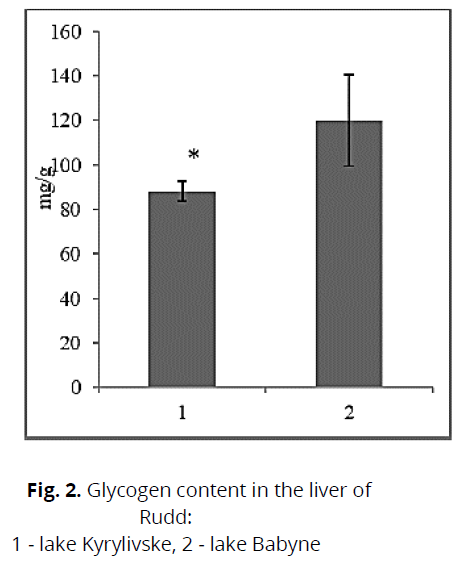
Fig 2: Glycogen content in the liver of Rudd:
1 - lake Kyrylivske, 2 - lake Babyne
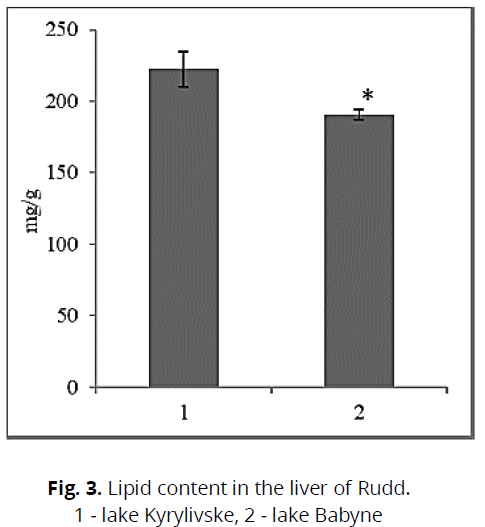
Fig 3: Lipid content in the liver of Rudd.
1 - lake Kyrylivske, 2 - lake Babyne
Note: * - reliability between samples (p≤0.05).
There was a significant difference in the content of malonic dialdehyde in the liver of Rudd in different reservoirs. In fish from the lake Kyrylivske (dirty reservoir), its content was higher by 40.7% (p≥0.05) compared to the same indicator for conditional control (Fig. 4).
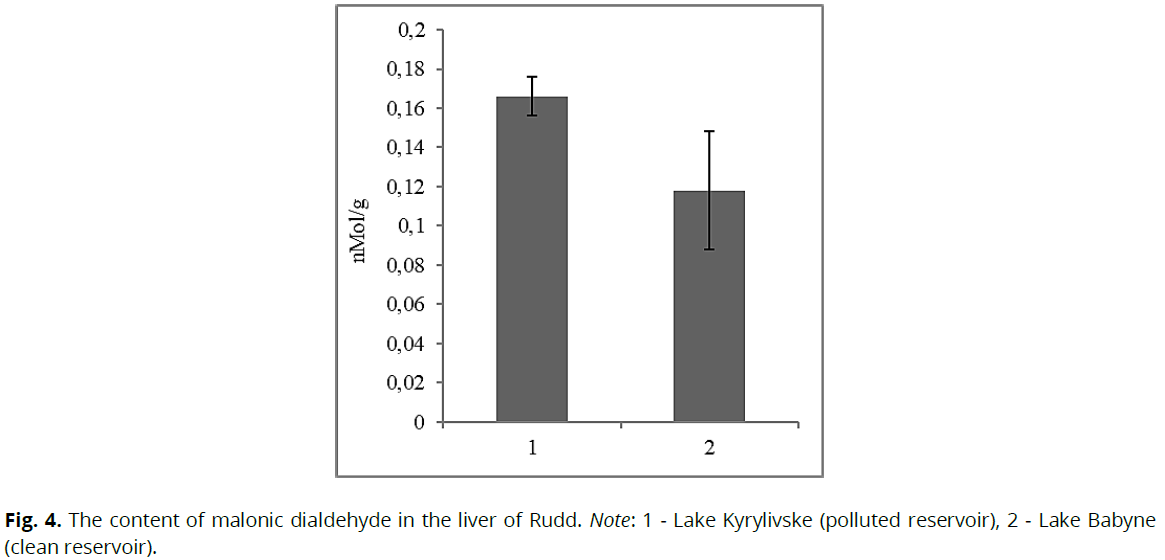
Fig 4: The content of malonic dialdehyde in the liver of Rudd. Note: 1 - Lake Kyrylivske (polluted reservoir), 2 - Lake Babyne (clean reservoir).
Malonic dialdehyde is one of the end products of peroxidation, and its accumulation in tissues indicates an increase in lipid peroxidation and the presence of stressful conditions for fish. We established that in the conditions of chronic water pollution, the content of malonic dialdehyde in the tissues of fish increases, indicates that the fish was in a state of chronic stress at the time of the study. Subsequently, we considered changes in the content of thyroid hormones. Thus, we found that the content of T3 in the blood plasma of fish under the influence of toxic contamination was 2.9 times lower (p≤0.05) (Fig. 5) relative to fish from control conditions. However, the content of T4 in the blood plasma of Rudd from the contaminated lake was higher by 40.0% (p≤0.05) relative to the conditional control (Fig. 6).
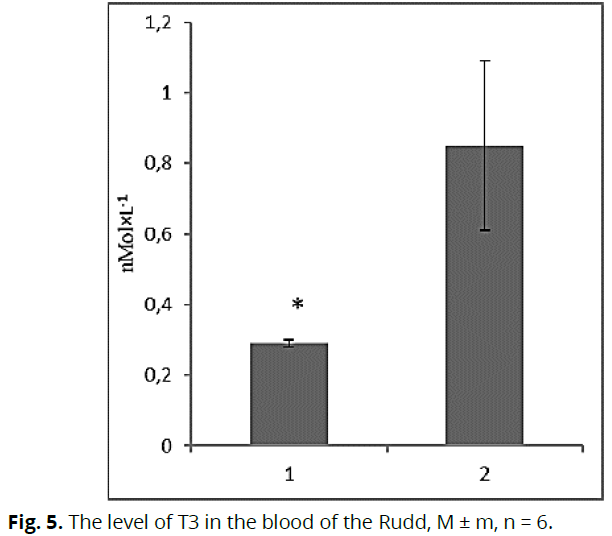
Fig 5: The level of T3 in the blood of the Rudd, M ± m, n = 6.
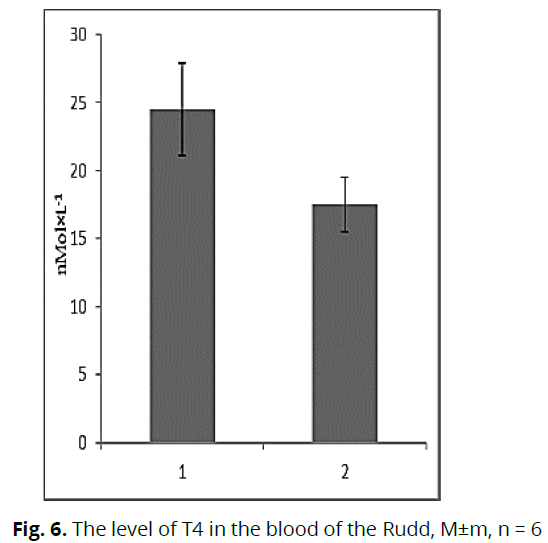
Fig 6: The level of T4 in the blood of the Rudd, M±m, n = 6
Note: 1 - Lake Kyrylivske (polluted reservoir), 2 - Lake Babyne (clean reservoir). * – reliability between samples (p≤0.05).
The neurohumoral system of fish is known to trigger and regulate the processes associated with adaptation to various factors. We investigated changes in the content of thyroid hormones and corticosteroids in the blood plasma of Rudd under the influence of water pollution. Thyroid hormones directly ensure the development of stress reactions in fish and activate the overall metabolism during adaptation (Peter et al., 2007; Peter et al., 2009). In the conditions of toxic pollution of reservoirs, the content of T3 in the blood of the Rudd was lower, and the content of T4 was higher concerning the fish from the conditional control. Kyrylivske is periodically contaminated with petroleum products (Romanenko et al., 2015), which can cause changes in the levels of these hormones. It is known that petroleum products affect the metabolic balance of fish and change T3 and T4 levels in the blood (Peter et al., 2007). Some authors note the role of thyroid hormones in regulating water-salt balance (Arjona et al., 2011, Peter, 2011). Changes in the total content of these hormones and their ratio may be due to an increase in the total salinity of water in the anthropogenically transformed reservoir. It is known that for the period of research in the lake Kyrylivske the content of chlorides and sulfates was much higher than similar indicators in the conditional control. This leads to increased metabolic processes in the body and increased oxygen consumption by fish. Considering that thyroid hormones are involved
in the regulation of lipid metabolism, the increase in total lipids (see Fig. 3) is due to the strengthening of compensatory processes by hormones, particularly T3. Thus, thyroid hormones reduce the harmful effects of toxicants on the body. Because of these changes in the environment, organisms exhibit a wide range of reactions to restoring homeostasis. Similar results have been demonstrated (Martseniuk et al., 2017). In addition to the above hormones, we considered the content of cortisol. The fish from the lake Kyrylivske (dirty pond) cortisol content in the blood plasma of fish was (p≥0.05) higher (by 27.2%) relative to fish from the conditional control (Fig. 7).
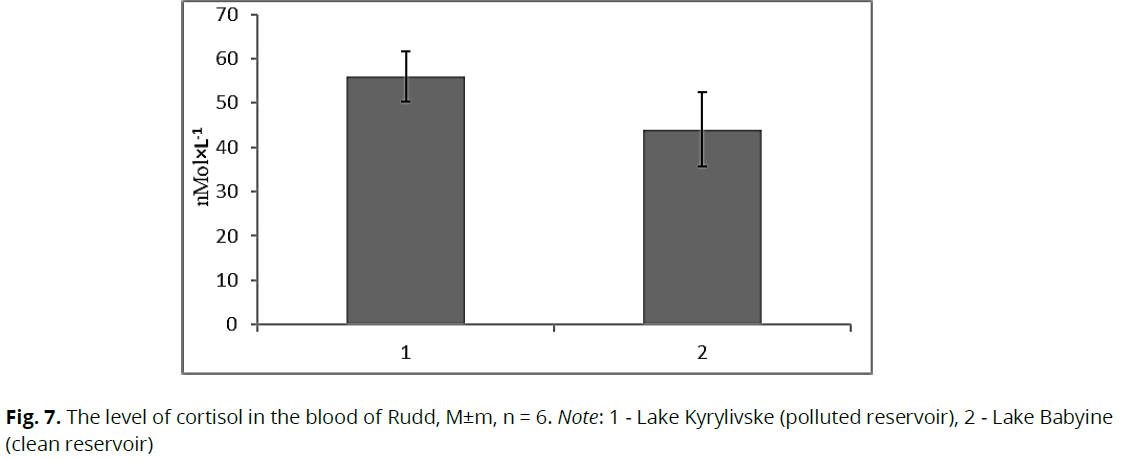
Fig 7: The level of cortisol in the blood of Rudd, M±m, n = 6. Note: 1 - Lake Kyrylivske (polluted reservoir), 2 - Lake Babyine (clean reservoir)
Cortisol is actively involved in the development and regulation of stress responses (Mommsen et al., 1999). High blood levels allow fish to save energy and return to a state of homeostasis after exposure to a stress agent (Sinha, 2012). A slight increase in cortisol content in the blood of fish from a polluted pond indicates their relative adaptation to living conditions. Considering the changes in the level of thyroid hormones in the blood plasma of Rudd, depending on the toxic pollution, they adequately reflect the ecological status of the studied reservoirs. The group of Rudd that has existed for a long time in the reservoir (Lake Kyrylivske, polluted reservoir), which is subject to periodic contamination with nitrogen compounds, petroleum products, heavy metals, has produced some adaptive reactions associated with changes in morpho-physiological parameters, which is directly related to changes in the level of thyroid hormones.
Conclusion
Thus, our research shows that the Rudd is a convenient test object for monitoring studies and assessing the degree of contamination of water bodies with toxicants. This species responds to the deterioration of reserve ecological status in terms of morpho-physiological and biochemical parameters. The most convenient indicators are changes in the indices of internal organs, the content of glycogen in the liver, and thyroid hormones in the blood. Thus, studies have shown that the fish from the contaminated lake had a triiodothyronine content 2.9 times higher than Rudd from the conditional control, indicating the strengthening of general metabolic processes. This was emphasized by a decrease in the content of glycogen and proteins in Rudd's liver tissues from the polluted lake, making it possible to conduct the biomonitoring of natural groups of fish in reservoirs subject to toxic pollution. Based on changes in physiological indicators, it is possible to predict the possible adverse effects of toxic compounds on groups of different species of fish.
References
Arjona, F.J., Vargas-Chacoff, L., Martin Del Rio, M.P., Flik, G., Mancera, J.M., & Klaren, P.H. (2011). Effects of cortisol and thyroid hormone on peripheral outer ring deiodination and osmoregulatory parameters in the Senegalese sole (Solea senegalensis). Journal of Endocrinology, 208, 323–330. doi: 10.1530/JOE-10-0416 Bashchenko, M.I., Boiko, О.V., Honchar, О.F., Gutyj, B.V., Lesyk, Y.V., Ostapyuk, A.Y., Kovalchuk, І.І., & Leskiv, Kh.Ya. (2020). The effect of milk thistle, metiphen, and
silimevit on the protein-synthesizing function of the liver of laying hens in experimental chronic cadmium toxicosis. Ukrainian Journal of Ecology, 10(6), 164– 168. doi: 10.15421/2020_276
Borshch, O.O., Borshch, O.V., Sobolev, O.I., Nadtochii, V.M., Slusar, M.V., Gutyj, B.V., Polishchuk, S.A., Malina, V.V., Korol, A.P., Korol-Bezpala, L.P., Bezpalyi, I.F., & Cherniavskyi, O.O. (2021). Wind speed in easily assembled premises with different design constructions for side curtains in winter. Ukrainian Journal of Ecology, 11 (1), 325–328. doi: 10.15421/2021_49
Borshch, O.O., Gutyj, B.V., Borshch, O.V., Sobolev, O.I., Chernyuk, S.V., Rudenko, O.P., Kalyn, B.M., Lytvyn, N.A., Savchuk, L.B., Kit, L.P., Nahirniak, T.B., Kropyvka, S.I.,
&Pundyak, T.O. (2020). Environmental pollution caused by the manure storage. Ukrainian Journal of Ecology, 10(3), 110–114. doi: 10.15421/2020_142 Butsiak, H.A., Butsiak, V.I., Gutyj, B.V., Kalyn, B.M., Muzyka, L.I., Stadnytska, O.I., Luchyn, I.S., Rozputnii, O.I., Kachan, L.M., Melnichenko, Yu.O., Sliusarenko, S.V.,
Bilkevich, V.V., & Leskiv, K.Y. (2021). Migration of mobile forms of heavy metals into the vegetative mass of plants under local human-caused load. Ukrainian Journal of Ecology, 11 (1), 239-343. doi: 10.15421/2021_50
Cicik, B., & Engin, K. (2005). The effects of cadmium on levels of glucose in serum and glycogen reserves in the liver and muscle tissues of Cyprinus carpio (L, 1758). Turkish journal of veterinary and animal sciences, 29, 113–117.
de La Torre, E.R., Ferrari, L.L., & Salibián, A. (2005). Biomarkers of a native fish species (Cnesterodon decemmaculatus) application to the water toxicity assessment of aperi-urbanpolluted river of Argentina.Chemosphere, 59(4), 577–583. doi: 10.1016/j.chemosphere.2004.12.039
Fedonenko, O.V., Filippova, E.V., & Sharamok, T.S. (2008). Otsinka rivnya zabrudnennya Zaporizʹkoho vodoskhovyshcha vazhkymy metalamy zasobamy roslyn. Naukovyy visnyk Uzhhorodsʹkoho universytetu (Seriya Biolohiya), 24, 100–103 (in Ukraine).
Fernandes, C., Fontaínhas-Fernandes, A., Rocha, E., & Salgado, M.A. (2008). Monitoring pollution in Esmorzi-Paramos lagoon, Portugal: liver histological and biochemical effects in Liza sapiens.Environmental Monitoring and Assessment, 145, 315–322. doi: 10.1007/s10661-007-0041-4
Guinan, M.E., Kapuscinski, K.L., & Teece, W.A. (2015). Seasonal diet shifts and trophic position of an invasive cyprinid, the rudd Scardinius erythrophthalmus (Linnaeus, 1758), in the upper Niagara River. Aquatic Invasions, 10(2), 217–225. doi: 10.3391/ai.2015.10.2.10
Honcharova, O.V., Paraniak, R.P., Kutishchev, P.S., Paraniak, N.M., Hradovych, N.I., Matsuska, O.V., Rudenko, O.P., Lytvyn, N.A., Gutyj, B.V., Maksishko, L.M. (2021). The influence of environmental factors on fish productivity in small reservoirs and transformed waters. Ukrainian Journal of Ecology, 11 (1), 176-180. doi: 10.15421/2021_27
Hori, T.S.F., Avilez, I.M., Inoue, L.A., & Moraes, G. (2006). Metabolical changes induced by chronic phenol exposure in matrinxã Brycon cephalus (teleostei: characidae)
juveniles. Comparative Biochemical Physiology, 143(1), 67–72. doi: 10.1016/j.cbpc.2005.12.004
Kalaitan, T.V., Stybel, V.V., Gutyj, B.V., Hrymak, O.Ya., Kushnir, L.P., Yaroshevych, N.B., Vovk, M.V., & Kindrat, O.V. (2021). Ecotourism and sustainable development. Prospects for Ukraine. Ukrainian Journal of Ecology, 11(1), 373-383. doi: 10.15421/2021_55
Kalyn, B.M., Khromova, M.V., Vishchur, V.Ia., Butsiak, H.A., Kropyvka, S.I., Gutyj, B.V. (2020). Estimation of quality of surface water of Dniester river basin within Lviv and Khmelnytsk regions. Ukrainian Journal of Ecology, 10(6), 127-132. doi: 10.15421/2020_271
Kofonov, K., Potrokhov, О., Hrynevych, N., Zinkovskyi, O., Khomiak, O., Dunaievska, O., Rud, O., Kutsocon, L., Chemerys, V., Gutyj, B., Fijalovych, L., Vavrysevych, J., Todoriuk, V., Leskiv, K., Husar, P., & Khumynets, P. (2020). Changes in the biochemical status of common carp juveniles (Cyprinus carpio L.) exposed to ammonium chloride and potassium phosphate. Ukrainian Journal of Ecology, 10(4), 137–147. doi: 10.15421/2020_181
Krishna, M.V., Bhaskar, M., & Govindappa, S.I. (1994). Studies on lipid profiles of fish liver on acclimation to acidic medium. Environmental biology, 15, 269–273. Kumar, S, Gaikwad, S.A., Shekdar, A.V., Kshirsagar, P.S., & Singh, R.N. (2004). Estimation method fornational methaneemission from solid wastel and fills.
Atmospheric Environment, 38(21), 3481–3487. doi: 10.1016/j.atmosenv.2004.02.057
Kuzminova, N.S. (2006). Indeks pecheni Chernomorskogo skadasana, indikator yeye fiziologicheskogo sostoyaniya. Rybolovstvo Ukrainy, 2(43), 36–38 (in Russian). Liebel, S., Tomatake, M.E.M., & Cico Alberto de Oliveira, R. (2013). Fish histopathology as biomarker to evaluate water quality. Ecotoxicology Environment
Contamination, 8(2), 9–15.doi: 10.5132/eec.2013.02.002
Lowry, O.H., Rosebrough, N.J., Farr, A.L., & Randall, R.J. (1951). Protein measurement with Folin phenol reagent. Journal of Biological Chemistry, 193(1951), 265–275 Martseniuk, V.M., Potrokhov, A.S., Zinkovskyi, & Prichepa, M.V. (2017). Physiological-biochemical status of perch and roach in conditions of excessive anthropogenic
pressure on the body of water. Fischeries Science of Ukraine, 4(42), 99–111. doi: 10.15407/fsu2017.04.099
Mommsen, T.P., Vijayan, M.M., & Moon, T.W. (1999). Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Reviews in FishBiology and Fisheries, 9, 211–268. doi: 10.1023/A:1008924418720
Novikov, Y., Lastochkina, K., & Boldina, Z. (1990). Metody isledovania kachestva vody vodoemov. Meditsyna, Moscow (in Russian).
Osoba, I.A. (2013). Biologicheskaya rol' perekisnogo okisleniya lipidov v funktsionirovanii organizma ryb. Rybovodnaya nauka Ukrainy, 1, 88–96 (in Ukrainian). Panasyuk, I.V., Tomilʹtseva, A.I., Zub, L.M., & Pohoryelova, YU.V. (2015). Yakistʹ vody u misʹkykh vodoymakh ta kharakter osvoyennya vodookhoronnykh zon (na
prykladi ozer systemy “Opechenʹ”, m.Kyyiv). Ekolohichna bezpeka ta pryrodokorystuvannya, 4(20), 63–69 (in Ukrainian).
Peter, M.C. (2011). The role of thyroid hormones in stress response of fish. General Comparative Endocrinology, 172(2), 198–210. doi: 10.1016/j.ygcen.2011.02.023 Peter, M.C.S., Joshya, E.K., Rejitha, V., & Peter, S.M.C. (2009). Thyroid hormone modifies the metabolic response of air-breathing perch (Anabas testudineus Bloch) to nimbecidine exposure. Journal Endocrinology. Reproduction, 1, 27–36. doi: 10.18311/jer/2009/2013
Peter, V.S., Joshua, E.K., Wendelaar Bonga, S.E., & Peter, S.M.C. (2007). Metabolic and thyroidal response in air-breathing perch (Anabastes tudineus) to water- bornekerosene, General and Comparative Endocrinology, 152(2-3), 198–205. doi: 10.1016/j.ygcen.2007.05.015
Pravdin, I.F. (1966). Spravochnik po izucheniyu ryb. Moskva, Rossiya, Pishchevaya promyshlennost' Press (in Russian).
Prychepa, M.V., Potrokhov, O.S., & Zin'kovskiy, O.G. (2019). Peculiarities of Biochemical Response of Fish to Anthropogenic Load under Conditions of Urbanization. Hydrobiological journal, 55(3), 44–52. doi: 10.1615/HydrobJ.v55.i3.50
Prychepa, M.V., Potrokhov, O.S., & Zinʹkovsʹkyy, O.H. (2017). Osoblyvosti zminy deyakykh biokhimichnykh pokaznykiv u riznykh ekolohichnykh hrup ryb za diyi antropohennoho navantazhennya. Naukovyy visnyk Chernivetsʹkoho universytetu. Biolohiya (Biolohichni systemy), 9(1), 39–43 doi: 10.31861/biosystems2017.01.039
Prysiazhniuk, N., Grynevych, N., Slobodeniuk, O., Kuzmenko, O., Tarasenko, L., Bevz, O., Khomiak, O., Horchanok, A., Gutyj, B., Kulyaba, O., Sachuk, R., Boiko, O., & Magrelo, N. (2019). Monitoring of morphological parameters of Cyprinidae liver. Ukrainian Journal of Ecology, 2019, 9(3), 162–167.
Romanenko, O.V., Arsan, O.M., Kipnis, L.S., & Sitnik, Yu.M. (2015). Ekologicheskiye problemy kiyevskikh vodoyemov i prilegayushchikh territoriy, Kiyev, Ukraina, Naykova dymka press (In Ukrainian).
Romanenko, V.D. (2006). Metody hidroekolohichnykh doslidzhen poverkhnevykh vod. K., 248–251 (in Ukrainian).
Rudenko, O.P., Paranjak, R.P., Kovalchuk, N.A., Kit, L.P., Hradovych, N.I., Gutyj, B.V., Kalyn, B.M., Sukhorska, O.P., Butsiak, A.A., Kropyvka, S.I., Petruniv, V.V., & Kovalska, L.M. (2019). Influence of seasonal factors on carp fish immune reactivity. Ukrainian Journal of Ecology, 2019, 9(3), 168–173.
Rudneva, I.I., & Kuzminova, N.S (2011). Izmeneniya nekotorykh vidov-markerov chernomorskikh ryb, obitayushchikh v usloviyakh khronicheskogo zagryazneniya. Ekologicheskiye sistemy i pribory, 2, 8–12 (in Russian).
Rudneva, N.N. (2006). Ispol'zovaniye biomarkerov ryb dlya ekologicheskoy diagnostiki vodnoy sredy. Ribne gospodarstvo Ukrainy, 42(1), 20–23 (in Ukrainian) Saranchuk, I.I., Vishchur, V.Ya., Gutyj, B.V., & Klim, O.Ya. (2021). Effect of various amounts of sunflower oil in feed additives on breast tissues' functional condition,
reproductivity, and productivity of honey bees. Ukrainian Journal of Ecology, 11 (1), 344-349. doi: 10.15421/2021_51 Sergeeva, N.R., & Lukyanenko, V.I. (2008). Obshchaya ikhtiotoksikologiya.Krasnodar: Nauka. 157.(in Russian)
Severin, S.Ye., & Solov'yev, G.A. (1989). Praktikum po biokhimii: Uchebnik. posobiye. Moskva, Ukraina, Izd-vo MGU (in Russian).
Shikhshabekov, M.N., Fedonenko, E.V., Marenkov, O.M., Abdulaeva, N.M., Rabazanov, N.I. (2000). Adaptive potential and functional features of fish reproductive systems in ecologically transformed reservoirs. Dnypropetrovsk, Zhurfond (in Ukrainian).
Sinha, A.K. (2012). Combined effects of high environmental ammonia, starvation and exercise on hormonal and ion-regulatory response in goldfish (Carassius auratus L.), Aquatic Toxicology, 114, 153–164. doi: 10.1016/j.aquatox.2012.02.027
Sole, M., Rodriguez, S., Papiol, V., Maynou, F., & Cartes, J.E. (2009). Xenobiotic metabolism markers in marine fish with different trophic strategies and their relationship to ecological variables. Comparative Biochemistry. Physiology, 149(1), 83–89. doi: 10.1016/j.cbpc.2008.07.008
Stalnaya, I., & Garishvili, T. (1977). Metod opredeleniya malonovogo dial'degida s ispol'zovaniyem tiobarbiturovoy kisloty. Sovremennyye metody v biologii, Moskva, Metodologiya, 66–68 (in Russian).
Vasylyev, D., Priimenko, B., Aleksandrova, K., Mykhalchenko, Y., Gutyj, B., Mazur, I., Magrelo, N., Sus, H., Dashkovskyy, O., Vus, U., & Kamratska, O. (2021). Investigation of the acute toxicity of new xanthine xenobiotics with noticeable antioxidant activity. Ukrainian Journal of Ecology, 11 (1), 315-318. doi: 10.15421/2021_47 Vatukuru, S. (2005). Acute effects of hexavalent chromium on survival, oxygen consumption, hematological parameters and some biochemical profiles of the Indian major carp, Labeorohita, Int. J. Environ Res. Public Health, 2, 456–462. doi: 10.3390/ijerph2005030010
Viana, A.P., & Lucena Fredon, F. (2009). Ichtyofauna as bioindicator of environmental quality in on industrial distinct in the amazon estuary. Brazil Brazilian Journal of Biology, 74(2), 315–324. doi: 10.1590/1519-6984.16012
Viarengo, A., Lowe, D.M., Bolognesi, C., & Fabri, E. (2007). The use of biomarkers in biomonitoring: A 2-tierapproachassessing the level of pollutantin duced stress syndrome insentinel organisms. Comp. Biochem. Physiol, 146, 281–300. doi: 10.1016/j.cbpc.2007.04.011
Yevtushenko, M.Yu., Dudnyk, S.V., & Hlyebova, Yu.A. (2007). Aklimatyzatsiya hidrobiontiv. Kyyiv. Ukrayina, Ahrarni nauky (in Ukrainian).
Zhezherya, V.A., Lynnyk, P.M., & Zubenko, I.B. (2016). Umist ta formy znakhodzhennya metaliv u ozerakh systemy Opechenʹ (m. Kyiv). Naukovi pratsi UkrNDHMI, 269, 70–86 (in Ukrainian).
Author Info
A. Sliusarenko2, H. Makhorin6, V. Prus6, L. Bozhyk5, L. Yevtukh6, V. Honcharenko6, P. Pukalo5, B. Gutyj5*, O. Dunaievska4, M. Prychepa1*, L. Kytsokon3, O. Rud3, O. Khomiak2, O. Vodianitskyi1, О. Potrokhov1, V. Martseniuk1 and N. Hrynevych22Bila Tserkva National Agrarian University, Bila Tserkva, Ukraine
3Rivne State Humanitarian University, Rivne, Ukraine
4Zhytomyr College of Pharmacy, Zhytomyr, Ukraine
5Stepan Gzhytskyi National University of Veterinary Medicine and Biotechnologies, Lviv, Ukraine
6Pollisia National University, Zhytomyr, Ukraine
Citation: Prychepa, M., Hrynevych, N., Martseniuk, V., Potrokhov, О., Vodianitskyi, O., Khomiak, O., Rud, O., Kytsokon, L., Sliusarenko, A., Dunaievska, O., Gutyj, B., Pukalo, P., Honcharenko, V., Yevtukh, L., Bozhyk, L., Prus, V., Makhorin, H. (2021). Rudd (Scardinius Erythrophthalmus l., 1758) as a bioindicator of anthropogenic pollution in freshwater bodies. Ukrainian Journal of Ecology, 11 (2), 253-260.
Received: 15-Mar-2021 Accepted: 23-Apr-2021 Published: 30-Apr-2021, DOI: 10.15421/2021_108
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
