Research - (2021) Volume 0, Issue 0
Seasonal dynamics of phytoplankton indicators of the Zaporizhzhia (Dnipro) reservoir phytoplankton of the Zaporozhye reservoir
Y. Nikolenko* and O. FedonenkoAbstract
At present, the Zaporizhzhia (Dnipro) reservoir is exposed to increased anthropogenic pressure, intensive blue-green algae blooming and persistent eutrophication processes. Being the primary component of the ecosystem, phytoplankton responds to such changes first. The analysis of seasonal dynamics of phytoplankton allows us to assess the state of the aquatic ecosystems and the impact of anthropogenic activity on water bodies. The studies on phytoplankton during the vegetative season of 2019 have revealed that there is a seasonal distribution of the phytoplankton by the number of species, abundance and biomass in the Zaporizhzhia (Dnipro) reservoir. However, blue-green algae remain the dominant group in the surface layer of water. The maximum and minimum values of Shannon index in May, August and September at different regions. Best and worst areas in terms of water quality based on Shanon index, abundance and biomass parameter were the Monastyrsky island and Samara Bay, respectively.
Keywords
Phytoplankton, zaporizhian (dnipro) reservoir, biomass; abundance, shannon index.
Introduction
Phytoplankton is a key indicator for assessing water quality, because it is the main producer in hydroecosystems, producing oxygen and organic substances through photosynthesis (Li et al., 2020). Monitoring, modelling, and forecasting phytoplankton groups are essential to areas under anthropogenic load, protected areas, and reservoirs. (Liu & Stevenson, 2017; Lv et al., 2014).
Eutrophication, and especially algal blooms have become increasingly severe in water bodies in recent years and caused periodic deterioration of water quality (Yan et al., 2020. Xiao, et al.,2011 Wang, et al.,2012, Ma, et al., 2015). This problem is particularly acute for reservoirs.
Cyanobacteria are the dominant group of phytoplankton in eutrophic freshwater bodies worldwide. They develop massively in shallow, warm and polluted water bodies with low oxygen content (Stotts et al., 1993; Oberholster, 2004; Khyzhniak, M.I., 2020), like Zaporizhzhia (Dnipro) reservoir.
During its existence, the ecosystem of the Zaporizhzhia reservoir has undergone a number of transformations caused by changes in the hydrological regime. Today, the reservoir is under the increased anthropogenic pressure. Pollution of the reservoir with industrial-related and domestic effluents containing mineral and organic substances, pesticides, petroleum products and radionuclides changes the habitat of hydrobionts. This affects their species composition and dynamics of quantitative indicators (Fedonenko, et al., 2012).
Since phytoplankton is sensitive to changes in external parameters, it is important to understand its dynamics in aquatic ecosystems (Rahmana & Hamidah., 2020). In addition, seasonal water quality analysis helps to assess the impact of anthropogenic activity on water bodies (Mishra, et al., 2019). However, detailed seasonal studies of phytoplankton in the water area of the Zaporizhzhia reservoir for a long period of time (Fedonenko & Nikolenko, 2019)
The purpose of the work: to trace the seasonal dynamics of the phytoplankton quantitative and qualitative indicators of the Zaporizhzhia (Dnipro) reservoir.
Materials and Methods
Zaporizhzhia (Dnipro) cascade reservoir is located within the territory of the Dnipro and Zaporizhia administrative regions of Ukraine. It is a multi-purpose reservoir. The length of the reservoir is 129.7 km; the minimum width is 0.6 km, the maximum width is 7.0 km, the average one is 3.2 km, and the area at the normal retaining level is 28,838 km2. According to V.I. Zhadin's classification, the Zaporizhzhya reservoir belongs to the plain-river in terms of genesis and location, to the channels in terms of configuration, medium-deep in terms of depth, and has a very large exchange in terms of water exchange (Fedonenko, et al., 2012).
The formation of the reservoir regime at the present stage is influenced by external factors, such as water runoff, amount of precipitation, anthropogenic load, as well as the rate of water exchange and internal processes. In most areas, water in the reservoir belongs to Class 3 in terms of quality, to category 4 (satisfactory), it is eutrophic, β-meso saprobic. Constantly increasing anthropogenic pressure causes significant changes in the chemical composition of water. (Hubanova, 2019; Dvoretskyi & Bajdak, 2017).
Samples of phytoplankton were collected with a Ruttner`s bathometer from the surface horizon (0.25 m) in plastic containers, every two weeks during the 2019 vegetative season (from April to October) at 5 sites along the riverbed of the Zaporizhzhia reservoir. These sites differ in hydrological and hydrochemical conditions (Fig. 1): Samara Bay, Festival Wharf, Monastyrskyi island, the entry of the Mokra Sura River and the lower part of the reservoir (near the Viyskove village).
Fig. 1. Scheme of the Zaporizhian (Dnipro) reservoir.
Samara Bay (48°5340.21 N; 35°1873.20 E) is characterized by weak flow and a large area of shallow water, which leads to phytoplankton blooms and causes stagnation. The hydroecological state of the Bay is determined by the influence of highly mineralized mine wastewater. The main polluting components of this wastewater are finely dispersed particles and heavy metals. (Kurchenko & Sharamok, 2017).
Festival Wharf is also a shallow area of the Zaporizhzhia reservoir. It suffers from the increased anthropogenic impact, in particular, due to the discharge of urban domestic wastewater with a high content of phosphates and nitrates. (Yakovenko, et al.,2017). The Monastyrsky island has been a stationary point of hydrobiological research for several centuries. It is characterized as conditionally clean and distant from major pollutants.
In the area of the entry of the Mokra Sura river, the hydrochemical and hydrobiological regime is significantly affected by: the complex of right-bank sewage treatment plants of the Dnipro city and the waters of the Mokra Sura river, which are constantly polluted by domestic, industrial and agricultural effluents (Fedonenko et al. 2016).
The lower part of the Zaporizhzhia reservoir near the Viyskove village is considered to be a relatively clean water zone in ecological terms, as it is located in the agricultural zone and does not experience direct industrial impact. It is the deepest part of the Zaporizhzhia reservoir, the maximum depth reaches 62.5 m, and the current speed does not exceed 0.5 m/s even during the spring flood. The shallow water area in the lower part of the reservoir is small (Kurchenko & Sharamok, 2017; Shapovalenko & Ananieva, 2019).
Fixation, concentration, and laboratory investigation of samples were performed in accordance with generally accepted hydrobiological methods (Arsan et al., 2006). The samples were fixed by 40% formalin with a ratio to sample volume 1:100, concentration of samples was performed by sedimentation. The phytoplankton composition was determined in Najott's chamber at × 100-400. Biomass was determined by the volume calculation method. Taxon names are given according to Raznoobrazie vodoroslei Ukraine, 2000. Statistical processing of the obtained results was carried out in accordance with generally accepted methods of variational statistics. The probability of differences between indicators was estimated using the Student's t-test at the significance level p <0.05.
Results
During the study period, phytoplankton of the Zaporizhzhia reservoir was represented by 76 species and intraspecific taxa belonging to 5 divisions: Chlorophyta (46), Bacillariophyta (16), Сyanophita (10), Euglenophyta (2), Chrysophyta (1), Ochrophyta (1) (Table 1). During the study period, the greatest average species diversity (fig. 2) was recorded in the area of the Monastyrsky island (18 s.t.). However, the maximum number of species was observed in August in the area of the entry of the Mokra Sura River (27 i.s.t.). The smallest number of species was 10 i.s.t., and averaged to 12 i.s.t., was recorded in the Samara Bay.
| S.No. | Species | The Sampling Points | ||||
|---|---|---|---|---|---|---|
| Samara Bay | Festival Wharf | Monastyrskyi Island | The Entry Of The Mokra Sura River | The Lower Part of The Reservoir | ||
| Chlorophyta | ||||||
| 1 | Actinastrum HantzschiiLagerh. | + | ||||
| 2 | Ancistodesmus acicularis (A. Br.) Korschik. | + | + | + | ||
| 3 | Ancistodesmus falcatus(Corda) Ralfs. | + | + | + | + | |
| 4 | Ankistrodesmus fusiformis Corda | + | + | + | ||
| 5 | Chlamydomonas ellipticaKorsch. | + | ||||
| 6 | Chlamydomonas monadinaStein. | + | ||||
| 7 | Chlorella vulgarisBeijer. | + | + | |||
| 8 | Closterium rostratumEhr. | + | ||||
| 9 | Coelastrum microporum Nag. in A.Br. | + | + | + | + | |
| 10 | Cosmarium margaritiferumMenegh. | + | + | |||
| 11 | Desmidium SwartziiAg. | + | + | |||
| 12 | Dictyosphaerium tetrachotomum Printz | + | + | + | + | |
| 13 | Eudorina elegans Ehr. | + | ||||
| 14 | Golenkinia radiata Chod. | + | + | + | + | |
| 15 | Mougeotia genuflexa (Dillw.) Ag. | + | + | |||
| 16 | Pandorina morumBory. | + | + | |||
| 17 | Paradoxia multisetaSwir. | + | ||||
| 18 | Pediastrum Boryanum (Turp.) Menegh. | + | + | |||
| 19 | Pediastrum duplex Meyen var. duplex | + | + | + | + | |
| 20 | Pediastrum duplex var. gracilimum W.et G.S.West | + | + | + | + | |
| 21 | Pediastrum simplexMeyen | + | + | + | + | |
| 22 | Pediastrum tetras(Ehr.) Ralfs | + | ||||
| 23 | Phacotus cocciferKorsch | + | ||||
| 24 | Tetraëdron minimum (A.Braun) Hansgirg | + | ||||
| 25 | Coenococcus planctonicusKorshikov | + | + | |||
| 26 | Scenedesmus acuminatus(Lagerh.) Chod. | + | + | + | + | |
| 27 | Scenedesmus acutus Meyen | + | + | |||
| 28 | Scenedesmus denticulatus Lagerh. | + | + | + | ||
| 29 | Scenedesmus dimorphus (Turpin) | + | + | + | ||
| 30 | Scenedesmus falcatus Chodat | + | + | + | ||
| 31 | Scenedesmus opoliensis P. Richt. | + | + | + | ||
| 32 | Scenedesmus quadricauda (Turp.) Breb. | + | + | + | + | |
| 33 | Schizochlamis gelatinosa A. Br. | + | + | + | ||
| 34 | Schroederia setigera(Schroed.) Lemm. | + | + | |||
| 35 | Sphaerocystis schroeteri Chodat | + | + | |||
| 36 | Selenastrum gracileReinsch | + | + | |||
| 37 | Siderocelis ornata(Fott) Fott | + | + | |||
| 38 | Spirogyra crassaKutz. | + | + | |||
| 39 | Staurastrum vestitum Ralfs. | + | ||||
| 40 | Staurastrum bacillare Bred | + | ||||
| 41 | Staurastrum tetracerum Ralfs ex Ralfs | + | + | |||
| 42 | Tetrachlorella ornata Korsch | + | + | |||
| 43 | Tetraëdron regulare Kützing | + | ||||
| 44 | Tetrastrum staurogeniaeforme (Schroed.) Lemm. | + | ||||
| 45 | Ulotrix zonata Kutz. | + | + | + | + | |
| 46 | Zygnema pectinatum (Ag.) Czurda | + | ||||
| Bacillariophyta | ||||||
| 47 | Amphora ovalis (Kutz.) Kutz. | + | + | + | ||
| 48 | Cocconeis disculus (Schumann) Cleve | + | + | |||
| 49 | Cyclotella sp. | + | + | + | ||
| 50 | Cymbella cistula (Hempr.) Grun. | + | + | |||
| 51 | Cymbella microcephala Grun. | + | ||||
| 52 | Cymbella ventricosa Kutz. | + | + | |||
| 53 | Eunotia praerupta Ehrenberg | + | ||||
| 54 | Fragilaria capucina Desm | + | + | + | ||
| 55 | Melosira granulate (Ehr.) Ralfs | + | + | |||
| 56 | Navicula cryptocephala Kutz. | + | + | |||
| 57 | Navicula radiosa Kutz. | + | + | |||
| 58 | Nitzschia gracilis Hant. | + | ||||
| 59 | Pinnularia major (Kutz.) Rabenh. | + | ||||
| 60 | Stephanodiscus Hantzschii (Ehr.) Grun. | + | + | + | ||
| 61 | Synedra acus Kutz. | + | ||||
| 62 | Synedra ulna (Nitzsch.) Ehr | + | ||||
| Cyanophyta | ||||||
| 63 | Anabaena flos-aque Lyngb.) Breb. | + | + | + | ||
| 64 | Anabaena spiroides Kleb. | + | + | + | + | + |
| 65 | Aphanizomenon flos-aquae (L.) Ralfs | + | + | + | + | + |
| 66 | Chroococcus turgidus (Kützing) Nägeli | + | + | + | + | |
| 67 | Merismopedia minima G. Beck ? G.Beck & Zahlbruckner | + | ||||
| 68 | Microcystis aeruginosa (Kützing) Kützing | + | + | + | + | + |
| 69 | Microcystis flos-aquae (Wittr) Elenk emend. Kom | + | + | + | + | |
| 70 | Nodularia spumigena Mert. | + | + | |||
| 71 | Oscillatoria limosa Ag. | + | + | + | + | |
| 72 | Oscillatoria tenuis Ag. | + | + | + | ||
| Euglenophyta | ||||||
| 73 | Euglena acus Ehr. | + | ||||
| 74 | Euglena granulate (Klebs) Schmitz | + | ||||
| Chrysophyta | ||||||
| 75 | Synura lapponica Scuja | + | ||||
| Ochrophyta | ||||||
| 76 | Vaucheria litorea C. Agardh | + | ||||
Table 1. List of phytoplankton species identified in different parts of the Zaporizhzhia reservoir.
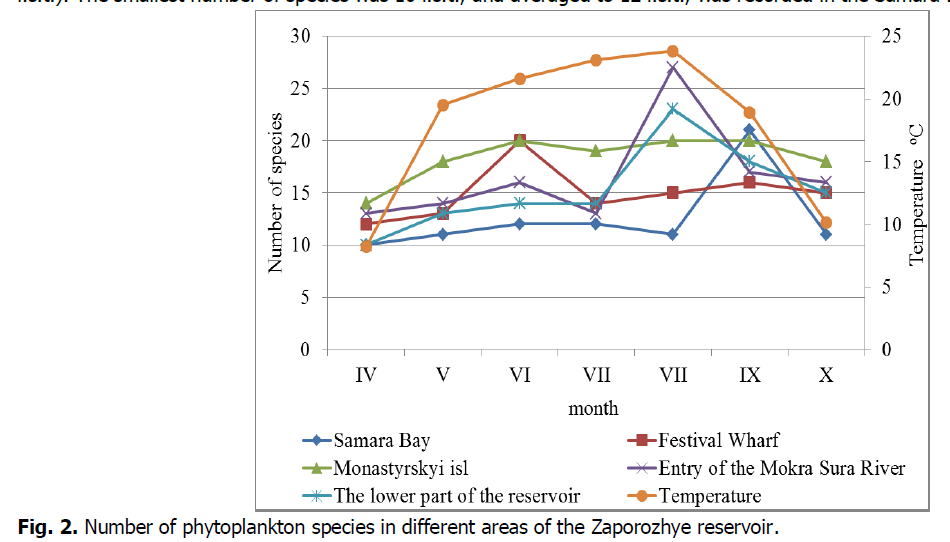
Figure 2: Number of phytoplankton species in different areas of the Zaporozhye reservoir
Seasonal dynamics were also traced by indicators of abundance and biomass (fig.3,4.). The maximum abundance at all selection points was recorded in August-early September and averaged 6.80 × 106 ± 21.6 × 106 cells L-1. The smallest abundance was recorded in October, it averaged 25.4 × 106 ± 40.8 × 106 cells L-1. As for the selection points, the highest abundance during the study period was observed in the Samara Bay–on average it was 61.4 × 106 ± 26.7 × 106 cells L-1, the lowest–in the area of Monastyrsky island–39.29 × 106 ± 15.2 × 106 cells L-1.
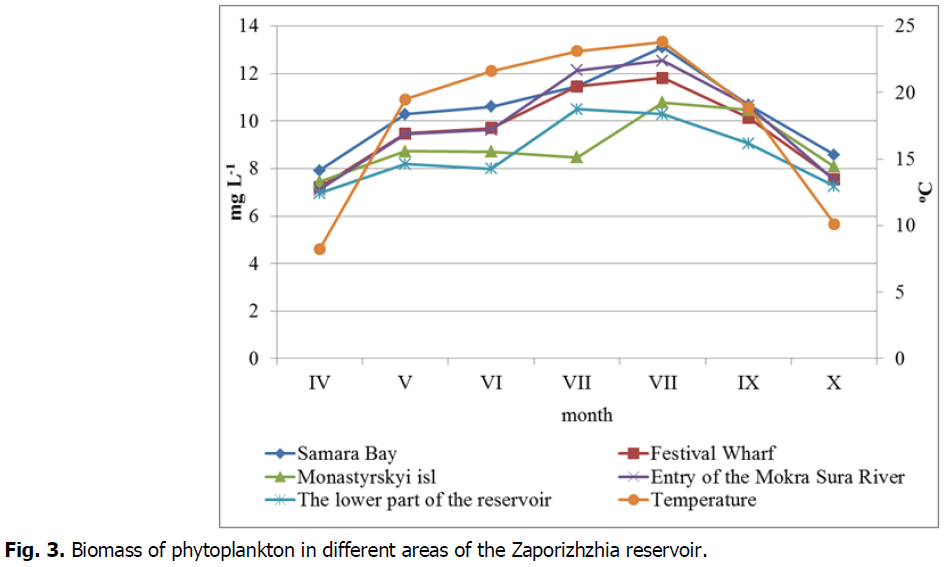
Fig. 3. Biomass of phytoplankton in different areas of the Zaporizhzhia reservoir.
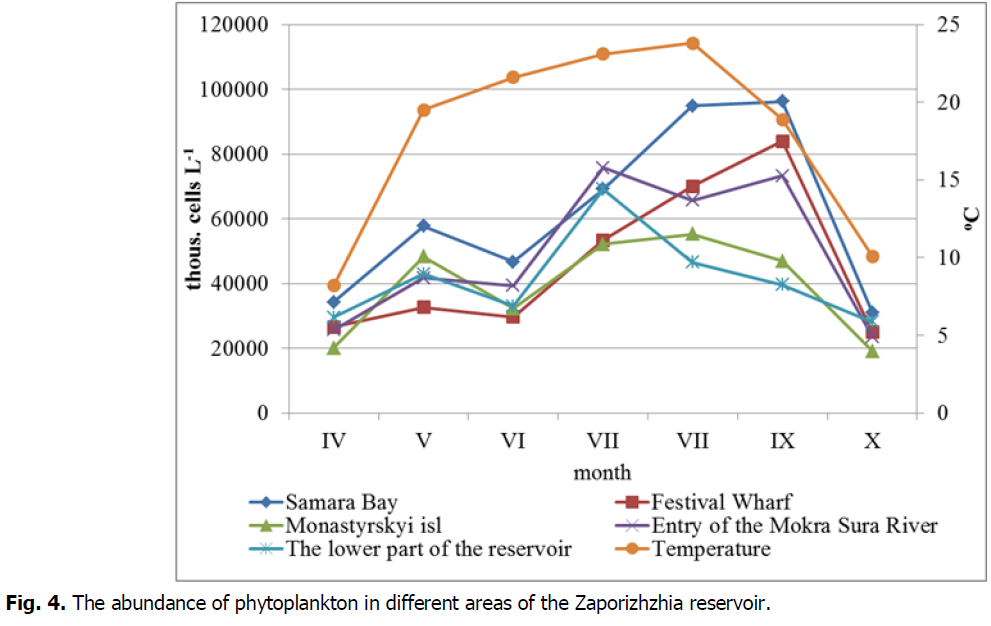
Fig. 4. The abundance of phytoplankton in different areas of the Zaporizhzhia reservoir.
Indicators of biomass are somewhat different. The maximum biomass values were recorded in August, they averaged 11.70 ± 1.051 mg L-1, while the minimum values were observed in April and amounted to 7.33 ± 0.339 mg L-1. The highest rates of phytoplankton biomass during the study period were observed in Samara Bay. They averaged 10.37 ± 1.73. mg L-1. The lowest values were recorded in the area of the Viyskove village, which averaged 8.74 ± 1.637 mg L-1.
During the sampling period, blue-green algae evidently dominated among phytoplankton groups, which is expressed in their percentage to the total phytoplankton abundance: from 68% in October in the Festival Wharf area, to 99% in August, in the Samara Bay. In relation to the total phytoplankton biomass, the situation varied: in the spring and summer period, the blue-green algae was a dominant group at most selection points. It reached from 34% in June, at the entry of the Mokra Sura River to 78% in July, near the Monastyrskyi island. However, it should be taken into account that green algae, along with blue-green algae, were also the dominant group at most selection points in the spring period, at the entry of the Mokra Sura River in June and in the lower part of the reservoir (the Viyskove village) in August. In the autumn period, diatoms and green algae occupied the dominant position in addition to blue-green algae at various selection points. Thus, in October, the proportion of green algae reached 61% of the total phytoplankton biomass in the area of the Festival Wharf, and in September, 41% and 44% of the total biomass consisted of representatives of diatoms on the lower section of the reservoir and Monastyrsky island respectively.
The basis of abundance and biomass was formed by the genera Microcystis Kütz, 1833, Aphanizomenon A. Morren ex Bornet & Flahault, 1888, Anabaena Bory ex Bornet and Flahault 1886, and to a lesser extent by Pediastrum Meyen, 1829, Scenedesmus Meyen, 1829 (Table 1).
It has been found that eutrophication often contributes to the mass blooming of several species that become highly dominant in phytoplankton groups. Therefore, taxonomically rich and diverse phytoplankton communities are considered indicators of good ecological status (Cozzoli et al., 2017).
Based on the aforesaid, during the study period, the Shannon index was determined in terms of the abundance and biomass of phytoplankton of the Zaporizhzhia reservoir (Table 2). On average, the highest values of phytoplankton biomass were recorded in the area of the Monastyrsky island (1.78 ± 0.369 bits) and the entry of the Mokra Sura River (1.80 ± 0.249 bits), the smallest were in the Samara Bay (1.41 ± 0.125 bits); in terms of abundance, the highest indicator (1.26 ± 0.241 bits) was recorded in the area of Monastyrsky Island, and the smallest one (0.88 ± 0.287 bits)–in the Samara Bay.
| The Sampling Points | April | May | June | July | August | September | October | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ? | N | B | N | ? | N | B | N | ? | N | B | N | ? | N | |
| Samara Bay | 1.42 | 1.22 | 1.29 | 1.11 | 1.3 | 1.03 | 1.44 | 0.63 | 1.4 | 0.47 | 1.66 | 0.66 | 1.44 | 1.04 |
| Festival Wharf | 1.52 | 1.21 | 1.32 | 1.14 | 1.92 | 1.33 | 1.77 | 0.78 | 1.6 | 0.62 | 1.22 | 0.38 | 1.62 | 1.05 |
| Monastyrskyi isl | 1.69 | 1.31 | 1.5 | 1.19 | 1.37 | 1.37 | 1.56 | 1.39 | 1.8 | 0.87 | 2.34 | 1.07 | 2.23 | 1.61 |
| Entry of the Mokra Sura River | 1.68 | 1.1 | 1.82 | 1.14 | 2.16 | 1.44 | 1.66 | 0.87 | 2.1 | 1.28 | 1.65 | 0.87 | 1.5 | 1.56 |
| The lower part of the reservoir | 1.45 | 1.19 | 1.44 | 1.17 | 1.48 | 1.24 | 1.9 | 0.93 | 1.8 | 1.05 | 1.78 | 0.78 | 1.61 | 0.93 |
| Bâ??The Shannon Index in terms of the biomass of phytoplankton. Nâ??The Shannon Index in terms of the abundance of phytoplankton. |
||||||||||||||
Table 2. Values of the Shannon index in different parts of the Zaporizhzhia reservoir.
Discussion
In general, there was an upward trend in the number of phytoplankton species from April to August-September, depending on the selection point and a decrease in October, which is mainly due to changes in water temperature. Thus, according to Rasconi et.al (2017) the temperature has the strongest effect on the spread of phytoplankton. However, it is worth noting that an abnormally high increase in temperature contributes to the growth of potentially toxic phytoplankton species and exacerbates the problem of blooming in water bodies and mass development of multicellular algae in the coastal zone (Golubkov, et.al, 2021; Lürling, et.al., 2017; Liu, et.al., 2021;. Jiang, et.al., 2014). Thus, an increase in nutrient input and temperature tend to mutually enhance the symptoms of eutrophication (Rahmana, et.al, 2020). The latter is particularly acute in the Samara Bay, where the shallow water area occupies up to 90% of the upper part of the Bay, stagnant phenomena are observed (Kurchenko & Sharamok, 2017; Shapovalenko & Ananieva, 2019), and the water temperature reached 25oC, which contributed to a decrease in biodiversity, with increased vegetation of Cyanophita in the surface layer. In addition, there are high concentrations of zinc and copper in the Samara Bay, which are the most toxic for most algae (Sharamok et al., 2019).
The increase in abundance and biomass in August is primarily due to a favourable set of conditions for their development, namely: an increase in water temperature (up to 25oC), the accumulation of high concentrations of biogenic elements in the water mass, which leads to increased vegetation of algae, especially representatives of Cyanophyta. So, taking into account our previous studies, in August, at various selection points, the content of ammonia ranged from 0.01 ± 0.0005 mg L-1 to 0.58 ± 0.0009 mg L-1, at the norm of 0.05 mg L-1; nitrites from 0.005 ± 0.00024 mg L-1 to 0.1 ± 0.004 mg L-1, at the norm of 0.1 mg L-1; nitrates from 0.27 ± 0.012 mg L-1 to 1.49 ± 0.059 mg L-1 at the norm of 2 mg L-1, phosphates from 0.01 ± 0.0004 mg L-1 to 0.38 ± 0.021, mg L-1 at the norm of 0.5 mg L-1. There also were high indicators of permanganate oxidizability, indicating the content of readily oxidizable organic substances. They reached more than 10.5 mg L-1, at the lower limit of the norm of 10 mg L-1.
During almost the entire study period, blue-green algae remained the dominant group, especially in the surface layer of water, causing blooms. It was especially acute from mid-July to mid-September in the Samara Bay and the area of the Festival Wharf.
The intensive development of algae in the Samara Bay and the area of the Festival Wharf is explained both by hydrological conditions and by an increased anthropogenic load in these areas. The latter increases eutrophication, which in turn contributes to the development of planktonic algae, a decrease in water transparency, and as a result, a deterioration in the trophic state of reservoirs.
To assess phytoplankton diversity, it is important to determine the Shannon index, which takes into account both the number and uniformity of taxa present in phytoplankton communities. The index increases with the number of taxa in the community and can theoretically reach very high values (Francet et. al., 2021).
The seasonal distribution of the Shannon Index in terms of the abundance and biomass of phytoplankton of the Zaporizhzhia reservoir is hardly traced, at different selection points, the maximum and minimum values were recorded mainly in different periods, which indicates increased anthropogenic pressure on the reservoir, high concentrations of biogenic and organic substances.
The value of the Shannon index ≥2 was recorded only for phytoplankton biomass: in September and October in the area of Monastyrsky island, as well as in June and August in the area of the entry of the Mokra Sura river, which indicates a polydominant complex of phytoplankton in this period, and therefore insignificant anthropogenic pressure, which does not lead or slightly leads to degradation of phytoplankton. The index value ≤1, recorded only in terms of the abundance of phytoplankton. From July to September it was recorded in the area of Samara Bay and Festival Wharf, in August in the area of Monastyrsky Island, and July-August in the entry of the Mokra Sura River and on the lower section of the reservoir, which indicates a monodominant complex of phytoplankton, and therefore high anthropogenic pressure on these areas (Arsan et. al., 2006).
Conclusion
Phytoplankton of the Zaporizhzhia reservoir is characterized by a seasonal distribution by the number of species, abundance and biomass. There was an upward trend in the quantitative indicators of phytoplankton from April to August-September, depending on the selection point and a decrease in October, which is mainly due to changes in water temperature. The worst results for all the studied indicators were observed in the Samara Bay and in the area of the Festival Wharf, which is primarily due to increased anthropogenic pressure. The best indicators were recorded in the area of the Monastyrsky island and the lower part of the reservoir, which can characterize them as relatively clean areas. During almost the entire study period, blue-green algae remained the dominant group. They are resistant to anthropogenic impact and in the summer-autumn period cause intensive blooming of water, which indicates an increased negative impact on the aquatic ecosystem of the Zaporizhzhia reservoir and the need for comprehensive system studies to take appropriate measures.
Acknowledgement
The authors sincerely thank all our reviewers. This study was funded by the Ministry of Education and Science of Ukraine.
References
Arsan, O.M., Davydov, O., Dyachenko, T., Yevtushenko, N., Zhukinskiy, V., Kirpenko, N., Kipnis, L. (2006). Hydroecological surface water dilution methods. Logos Press.
Cadotte, M.W., Carscadden, K., Mirotchnick, N. (2011). Beyond species: Functional diversity and the maintenance of ecological processes and services. Journal of Applied Ecology, 48:1079-1087.
Cozzoli, F., Stanca, E., Selmeczy, G.B., Francé, J., Varkitzi, I., Basset, A. (2017). Sensitivity of phytoplankton metrics to sample-size: A case study on a large transitional water dataset (WISER). Ecological Indicators, 82:558-573.
Dvoretskyi, A.I., Bajdak, L.A. (2017). Peculiarities of the transformation of aquatic ecosystems of the Dniprovskoho Vodoshovishcha. Ruta, Zhytomyr.
Fedonenko, O.V., Nikolenko, Yu. (2019). Characteristics of the phytoplankton of the Zaporizkoho water reserve for the period of the season (uhlyad). Rybohospodarska Nauka Ukrainy, 2:21-41.
Fedonenko, O.V., Yesipova, N., Sharamok, T., Ananieva, T., Yakovenko, V., Zhezheria, V. (2012). Happy problems of nidrobiology. Lira Press.
Fedonenko, O.V. Ananeva, T.V. Nikolenko, Yu.V. (2016). Eco-column galvanization of the water brightness of the Mokra Sura river for the hydrochemical indicators. Hidrolohiia, Hidrokhimiia i Hidroekolohiia, 4:74-81.
Francé, J., Varkitzi, I., Stanca, E., Cozzoli, F., Skejić, S., Ungaro, N., Basset, A. (2021). Large-scale testing of phytoplankton diversity indices for environmental assessment in Mediterranean sub-regions (Adriatic, Ionian and Aegean Seas). Ecological Indicators, 126:107630.
Golubkov, S.M. (2021). Effect of climatic fluctuations on the structure and functioning of ecosystems of continental water bodies. Contemporary Problems of Ecology, 14:1-10.
Hubanova, N.L. (2019). Production of zoobenthos in various areas of the Dnipro (Zaporizhzhia) reservoir. Agrology, 2:156-160.
Jiang, Y.J., He, W., Liu, W.X., Qin, N., Ouyang, H.L., Wang, Q.M., Xu, F.L. (2014). The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecological Indicators, 40:58-67.
Khyzhniak, M.I., Rudyk-Leuska, N.Y., Yevtushenko, N.Y., Leuskyi, M.V., Dudnyk, S.V., Danchuk, O.V., Dumych, O.Y. (2020). Development and structure of phytoplankton in the middle part of Kremenchug reservoir. Ukrainian Journal of Ecology.
Kurchenko, V.O., Sharamok, T.S. (2017). Peculiarities of the histolonic structure of the ziaber deyakykh koropovykh fishes. Biolohichni Systemy, 9:70-74.
Li, Y., Meng, J., Zhang, C., Ji, S., Kong, Q., Wang, R., Liu, J. (2020). Bottom-up and top-down effects on phytoplankton communities in two freshwater lakes. PloS One, 15:e0231357.
Liu, C., Sun, X., Su, L., Cai, J., Zhang, L., Guo, L. (2021). Assessment of phytoplankton community structure and water quality in the Hongmen Reservoir. Water Quality Research Journal, 56:19-30.
Liu, B., Stevenson, R.J. (2017). Improving assessment accuracy for lake biological condition by classifying lakes with diatom typology, varying metrics and modeling multimetric indices. Science of the Total Environment, 609:263-271.
Lürling, M., Van Oosterhout, F., Faassen, E. (2017). Eutrophication and warming boost cyanobacterial biomass and microcystins. Toxins, 9:64.
Lv, J., Wu, H., Chen, M. (2011). Effects of nitrogen and phosphorus on phytoplankton composition and biomass in 15 subtropical, urban shallow lakes in Wuhan, China. Limnologica, 41:48-56.
Ma, W.X., Huang, T.L., Li, X., Zhang, H.H., Ju, T. (2015). Impact of short-term climate variation and hydrology change on thermal structure and water quality of a canyon-shaped, stratified reservoir. Environmental Science and Pollution Research International, 22:18372-18380.
Mishra, P., Garg, V., Dutt, K. (2019). Seasonal dynamics of phytoplankton population and water quality in Bidoli reservoir. Environmental Monitoring and Assessment, 191:130.
Oberholster, P.J., Botha, A.M., Grobbelaar, J.U. (2004). Microcystis aeruginosa: Source of toxic microcystins in drinking water. African Journal of Biotechnology, 3:159-168.
Rahman, M.M., Hamidah, H. (2020). Water quality influence the phytoplankton and bacteria abundance: A comparison between shallow freshwater and saltwater ponds. Desalin Water Treat, 188:436-443.
Rasconi, S., Winter, K., Kainz, M.J. (2017). Temperature increase and fluctuation induce phytoplankton biodiversity loss–Evidence from a multi‐seasonal mesocosm experiment. Ecology and Evolution, 7:2936-2946.
Raznoobrazie Vodoroslei Ukrainy (2000). Pod red.: Vassera S.P., Tcarenko P. Algologiia, 10:309. Shapovalenko, Z.V., Ananieva, T.V. (2019). Levels of dose-forming radionuclides in young perch (Perca fluviatilis) of Zaporizhia Reservoir. Biological systems, 11:161-166.
Sharamok, T.S., Fedonenko, O., Kurchenko, V., Nikolenko, Yu. (2019). Nidroecolonic galvanized zinc. Pytannia Bioindykatsii ta Ekolohii, 24:147-161.
Stevenson, J. (2014). Ecological assessments with algae: A review and synthesis. Journal of Phycology, 50:437-461.
Stotts, R.R., Namikoshi, M., Haschek, W.M., Rinehart, K.L., Carmichael, W.W., Dahlem, A.M., Beasley, V.R. (1993). Structural modifications imparting reduced toxicity in microcystins from Microcystis spp. Toxicon, 31:783-789.
Wang, S., Qian, X., Han, B.P., Luo, L.C., Hamilton, D.P. (2012). Effects of local climate and hydrological conditions on the thermal regime of a reservoir at Tropic of Cancer, in Southern China. Water Research, 46:2591-2604.
Yakovenko, V., Melnik, S., Fedonenko, E. (2017). Species composition, seasonal dynamics and distribution of phytoplankton of the zaporizke reservoir. International Letters of Natural Sciences, 62:1-10.
Yan, M., Chen, S., Huang, T., Li, B., Li, N., Liu, K., Zong, R., Miao, Y., Huang, X. (2020). Community compositions of phytoplankton and eukaryotes during the mixing periods of a drinking water reservoir: Dynamics and interactions. International Journal of Environmental. Research and Public Health, 17:1128.
Author Info
Y. Nikolenko* and O. FedonenkoCitation: Nikolenko, Y., Fedonenko, O. (2021). Seasonal dynamics of phytoplankton indicators of the Zaporizhzhia (Dnipro) reservoir phytoplankton of the Zaporozhye reservoir. Ukrainian Journal of Ecology. 11 (7), 121-128.
Received: 04-Aug-2021 Accepted: 20-Sep-2021 Published: 27-Sep-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
