Research - (2022) Volume 12, Issue 4
Sexual dimorphism and geographic variation in the bridled skink, Heremites vittatus (Oliver, 1804), (Sauria: Scincidae) in Anatolian Peninsula
M.K. Sahin1*, E.Y. Caynak2,3, Y. Kumlutas2,3, K. Candan2,3 and C. Ilgaz2,3Abstract
Skinks are the largest family of lizards; therefore, its species are widely throughout the world and especially diverse in Asia. However, the sexual dimorphism and geographic variation patterns were less studied in the western part of Asia continent. The Bridled Skink, Heremites vittatus, was examined in relatively narrow scale geography under these terms up to now. In this study, we used 27 metric and meristic characters from 210 specimens to assess sexual dimorphism and geographic variation patterns in the Anatolian Peninsula for the first time. It is known that Anatolia is composed with the joint of Arabian Plate’s northern edge to main Anatolid formations. Therefore, analysis was done to test the differences between Anatolian and Arabian populations. The results showed that Arabian females are larger than males, moreover the significant differences were observed in head size and limb characteristics are female-biased, too. Additionally, some characters, like NED, FIL, IPW and PW vary geographically, that might have an effect on natural selection to lead into genetic differentiation.
Keywords
Scincidae, Heremites vittatus, Anatolia, Arabian plate, metric character, meristic character, morphological difference.
Introduction
Body size variation was used to be explained as an example of adaptative geographic variation that within species tendency for increasing body size was closely related with the effect of decreasing environmental temperature or increasing latitude (Mayr, 1956). Other possible sources that may also explain this variation trait are seasonality (Ashton et al., 2007), flow regimes (Rivera, 2008), predation interactions (Stoner and Breden, 1988; Kroeker et al., 2016) and even anthropogenic effects (Wallace and Saba, 2009).
Sexual dimorphism (SD) is an important phenotypic trait for comparing sexes in animals and recently geographic variation (GV) contributes to the impact of this trait with spatial scale (Cruz-Elizalde et al., 2017; DeGregorio et al., 2018). Morphometric studies, based on body size, body shape, scalation in lizards not only provides information for understanding their taxonomic relationships, but also contributes to interpreting the results from biogeography studies (Bager et al., 2016). On the other hand, observed sexual dimorphism can be the evidence of either sexual selection (when males are larger than females) (Kwiatkowski and Sullivan 2002) or fecundity selection (when females are larger than males) (Roitberg et al., 2015).
With more than 1600 species, skinks are the largest family of lizards (Pianka and Vitt 2003; Uetz et al., 2022). Due to molecular phylogenetic studies in the last two decades, banded skink’s name has been changing: It was formerly known as Mabuya vittata (Greer, 1977), then Mabuya genus has come to Trachylepis (Mausfeld et al., 2000) and finally it is assessed with the genus name, Heremites (Karin et al., 2016). According to recent literature, this scincid lizard has been studied in herpetological survey records (Mulder, 1995; Kumlutaş et al., 2015), local morphological analysis (Budak, 1973; Baran, 1977; Ozdemir et al., 2001), male reproductive system (Nassar and Hraoui-Bloquet, 2014) and female reproductive cycle examinations (Nassar et al., 2013), ecological niche modelling (Fattahi et al., 2014a), molecular phylogeny (Güçlü et al., 2014; Karin et al., 2016), local sexual dimorphism research with partial geographic variations (Rastegar-Pouyani and Fattahi, 2015) and regional comparison between Turkey and Iran (Rastegar-Pouyani et al., 2021).
The Anatolian Peninsula has been composed of diverse continental fractions which were united into a single landmass in the late Tertiary (Okay, 2008). Pontides, Anatolids, Taurids and Arabian plate are the major formations in this land and climatic conditions and vegetation differentiations have been shaped with the contribution of this geologic aspect (Şenkul and Doğan, 2013). Arabian Plate’s joining to Anatolia Peninsula is slightly different than the other explained formations (Çıplak, 2004). In the present study, we undertake a detailed morphological study of specimens from different populations of H. vittatus to answer the following questions: i) is there any sexual dimorphism between sexes, ii) if so, does this differentiation reflect to geographic variation between main Anatolian and Arabian plate’s Anatolian part?.
Materials and Methods
The specimens were collected from different localities in the Anatolian Peninsula between 1994 and 2014 (Fig. 1). Only adult specimens were used in this study (Fig. 2). The exact locality of the specimens was determined using a GPS. They were incorporated into the collection of ZDEU (Zoology Department of Ege University) and are deposited in the Zoology Lab. of the Department of Biology at Faculty of Science, Dokuz Eylül University. Mensural, meristic, and qualitative data were recorded following the system of (Basoğlu and Baran, 1977; Ozdemir et al., 2001; Rastegar-Pouyani and Fattahi, 2015). The determination of the sex was demonstrated by direct visualization of hemipenes in males.
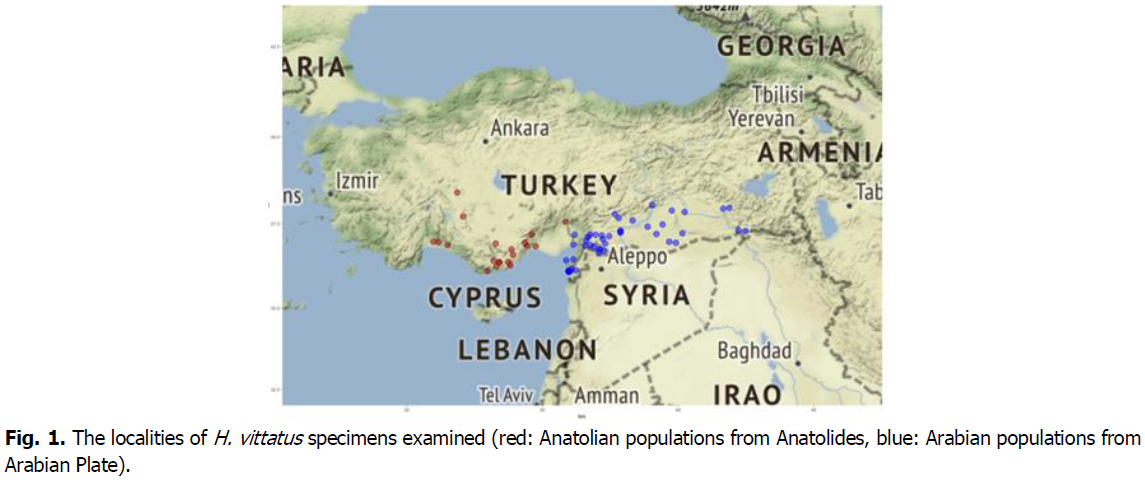
Fig 1. The localities of H. vittatus specimens examined (red: Anatolian populations from Anatolides, blue: Arabian populations from Arabian Plate).

Fig 2. Male (left) and female (right) H. vittatus from Diyarbakır, Southeastern Anatolia.
All measurements were determined under a stereo microscope. Metric measurements were recorded using a digital calliper with an accuracy of 0.01 mm. The following metric measurements were taken: SVL (snout-vent length), from tip of snout to anterior edge of cloaca; HL (head length), from tip of snout to anterior edge of tympanum; HW (Head width), at the widest point of head; HH (Head height), from lower edge infralabial to tip of supraocular; FLL (Forelimb length), outstretched limb from shoulder joint to tip of finger; HLL (Hindlimb length), outstretched limb from hip joint to tip of finger; EL (Eye length), from anterior corner to posterior corner of eye; NED (Nostril-eye distance), from anterior corner of eye to posterior edge of nostril; EED (Eye-ear distance), from the posterior corner of eye to anterior edge of tympanum; IORD (Interorbital distance), between anterior corner of orbit; SW (Snout width); TD (Tympanum diameter)-largest size; TOL (Fourth finger length); FIL (Fourth finger length); MSOL (Maximum length of subocular); FIPL (Length frontal to interparietal), from anterior corner of frontal to posterior corner of interparietal; IPW (Width of interparietal); PW (Parietal width) and PL (Parietal length).
Meristic (pholidosis) characteristics are following: subdigital lamellae under the fourth finger (SDLT), subdigital lamellae under the fourth finger (SDLF), number of supralabials (NSL), number of infralabials (NIL), number of scale rows around mid-body (NDS), number of ventral scales, from gular to anterior edge of cloaca (NVS), row of ventral scales (in longitudinal rows-RVS) and number of scales between posterior corner of eye to tip of ear (NEE). For all bilateral characters, only the left ones of the body were examined.
Metric and meristic raw data were firstly examined with Kolmogorov-Smirnov Test and equivalence of variances with Levene’s Test whether they met the requirements of normality or not (Zar, 2010). The metric and meristic data deviated from normality (Kolmogorov-Smirnov Test) were tested with the non-parametric Mann Whitney U Test for evaluating the differences between sexes and between populations (Zar, 2010). Morphometric variables were tested with analysis of variance (ANOVA) using sex (Male/Female) and population (Anatolian/Arabian) as grouping factors. Pairwise comparisons in each weak characteristic were done with a pooled one-way ANOVA (4 groups) and Tukey’s HSD was applied for post-hoc significance. Morphometric variables which were significant in ANOVA and showed sexual dimorphism pattern or geographic variation were used in a principal component analysis (PCA) to summarize the variability in morphometric data in order to exclude among-variables correlation. The linear discriminant analysis was used to compare the geographic variation in morphology among sexes. All these statistical analyses were carried out in R Software version 3.6 (R. Core Team 2018). p=0.05 was determined as significance level for the tests.
Results
In total morphometric measurements of 210 specimens were analyzed (the number of specimens from Anatolia is 52 (♂: 20, ♀: 32), the number from Arabian Plate is 158 (♂: 63, ♀: 95). The comparative morphometric data for females and males from both populations of H. vittatus is given in Table 1. According to normality test results, 5 of 27 morphological characters deviated from normality: MSOL, NSL, NIL, RVS and NEE.
| Anatolian | Arabian | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males (N=21) | Females (N=32) | Males (N=63) | Females (N=95) | ANOVA | ||||||||||||||
| Mean | St. Dev. |
Min | Max | Mean | St. Dev. |
Min | Max | Mean | St. Dev. |
Min | Max | Mean | St. Dev. |
Min | Max | F | p | |
| SVL | 69.65 | 7.87 | 57.82 | 89.13 | 74.86 | 8.73 | 61.87 | 89.34 | 71.02 | 6.96 | 57.52 | 84.41 | 76.41 | 9.12 | 57.95 | 96.81 | 7.222 | 0.000 |
| HL | 13.29 | 0.79 | 11.70 | 14.57 | 13.38 | 1.02 | 11.67 | 15.39 | 13.79 | 0.98 | 11.69 | 15.40 | 13.80 | 1.00 | 11.48 | 15.57 | 2.775 | 0.042 |
| HW | 7.57 | 0.84 | 6.13 | 9.51 | 7.80 | 0.95 | 6.09 | 10.20 | 7.90 | 0.80 | 6.20 | 9.36 | 7.90 | 0.85 | 5.99 | 9.66 | 0.905 | 0.440 |
| HH | 6.03 | 0.68 | 4.99 | 7.44 | 6.21 | 0.73 | 4.89 | 7.93 | 6.24 | 0.63 | 5.11 | 7.33 | 6.25 | 0.67 | 4.72 | 7.72 | 0.597 | 0.617 |
| FLL | 15.60 | 1.31 | 13.60 | 18.05 | 16.07 | 1.71 | 12.66 | 19.20 | 16.37 | 1.33 | 13.65 | 19.10 | 16.79 | 1.54 | 12.98 | 20.26 | 4.641 | 0.004 |
| HLL | 23.72 | 2.03 | 20.56 | 26.71 | 24.74 | 2.31 | 20.73 | 28.99 | 24.94 | 2.30 | 19.64 | 28.98 | 25.63 | 2.38 | 20.00 | 30.90 | 4.362 | 0.005 |
| EL | 3.85 | 0.45 | 2.65 | 4.58 | 3.86 | 0.47 | 3.02 | 4.74 | 3.85 | 0.44 | 2.84 | 4.77 | 3.86 | 0.46 | 2.61 | 4.91 | 0.017 | 0.997 |
| NED | 3.11 | 0.34 | 2.42 | 3.76 | 3.21 | 0.37 | 2.53 | 3.83 | 3.37 | 0.35 | 2.73 | 4.51 | 3.42 | 0.45 | 2.73 | 4.71 | 4.639 | 0.004 |
| EED | 3.59 | 0.37 | 2.75 | 4.09 | 3.65 | 0.39 | 2.90 | 4.50 | 3.80 | 0.52 | 2.78 | 5.39 | 3.84 | 0.53 | 2.74 | 5.71 | 2.099 | 0.102 |
| IORD | 4.84 | 0.54 | 3.82 | 5.89 | 4.93 | 0.55 | 3.91 | 6.21 | 5.07 | 0.50 | 4.00 | 6.24 | 5.10 | 0.49 | 3.89 | 6.23 | 0.353 | 0.787 |
| SW | 3.69 | 0.41 | 3.16 | 4.56 | 3.75 | 0.34 | 3.15 | 4.43 | 3.79 | 0.40 | 3.05 | 4.56 | 3.74 | 0.42 | 2.74 | 4.60 | 0.923 | 0.430 |
| TD | 1.51 | 0.26 | 1.10 | 2.13 | 1.44 | 0.26 | 0.84 | 1.86 | 1.52 | 0.27 | 1.04 | 2.19 | 1.52 | 0.23 | 1.14 | 2.11 | 0.923 | 0.430 |
| TOL | 8.14 | 0.48 | 6.86 | 8.84 | 8.24 | 0.67 | 7.02 | 9.56 | 8.32 | 0.80 | 6.45 | 10.14 | 8.54 | 0.81 | 6.67 | 10.47 | 2.553 | 0.057 |
| FIL | 4.53 | 0.54 | 3.68 | 5.82 | 4.34 | 0.53 | 3.14 | 5.40 | 4.53 | 0.46 | 3.10 | 5.86 | 4.62 | 0.52 | 2.84 | 5.77 | 2.467 | 0.063 |
| FIPL | 7.45 | 0.59 | 6.48 | 8.35 | 7.53 | 0.53 | 6.56 | 8.42 | 7.58 | 0.58 | 6.47 | 8.95 | 7.63 | 0.67 | 6.18 | 9.81 | 0.559 | 0.643 |
| IPW | 1.73 | 0.19 | 1.31 | 2.06 | 1.80 | 0.25 | 1.35 | 2.51 | 1.88 | 0.22 | 1.40 | 2.39 | 1.92 | 0.24 | 1.41 | 2.69 | 5.132 | 0.002 |
| PW | 3.05 | 0.27 | 2.49 | 3.39 | 3.14 | 0.40 | 2.33 | 4.14 | 3.38 | 0.36 | 2.34 | 4.34 | 3.41 | 0.34 | 2.61 | 4.12 | 8.933 | 0.000 |
| PL | 2.49 | 0.29 | 2.05 | 3.13 | 2.58 | 0.29 | 2.05 | 3.10 | 2.56 | 0.23 | 1.95 | 2.98 | 2.55 | 0.27 | 1.79 | 3.20 | 0.496 | 0.685 |
| MSOL | 2.38 | 0.23 | 1.97 | 2.76 | 2.37 | 0.22 | 1.95 | 2.82 | 2.37 | 0.23 | 1.90 | 2.88 | 2.36 | 0.26 | 1.76 | 2.88 | - | - |
| SDLT | 16.80 | 1.20 | 14.00 | 19.00 | 16.38 | 0.83 | 15.00 | 18.00 | 16.56 | 0.89 | 15.00 | 18.00 | 16.54 | 0.94 | 14.00 | 18.00 | 0.845 | 0.471 |
| SDLF | 11.65 | 0.88 | 10.00 | 13.00 | 11.50 | 0.67 | 10.00 | 13.00 | 11.37 | 0.77 | 10.00 | 13.00 | 11.38 | 0.80 | 10.00 | 13.00 | 0.881 | 0.451 |
| NSL | 7.00 | 0.00 | 7.00 | 7.00 | 6.94 | 0.35 | 6.00 | 8.00 | 7.00 | 0.00 | 7.00 | 7.00 | 7.00 | 0.25 | 6.00 | 8.00 | - | - |
| NIL | 6.95 | 0.39 | 6.00 | 8.00 | 7.00 | 0.25 | 6.00 | 8.00 | 7.00 | 0.00 | 7.00 | 7.00 | 7.02 | 0.25 | 6.00 | 8.00 | - | - |
| NDS | 31.40 | 0.88 | 30.00 | 32.00 | 31.75 | 0.98 | 30.00 | 34.00 | 31.90 | 1.03 | 30.00 | 34.00 | 32.13 | 1.02 | 30.00 | 34.00 | 3.406 | 0.019 |
| NVS | 41.40 | 1.73 | 37.00 | 45.00 | 42.22 | 1.86 | 39.00 | 47.00 | 42.38 | 1.75 | 39.00 | 47.00 | 42.65 | 1.87 | 39.00 | 48.00 | 2.725 | 0.045 |
| RVS | 8.50 | 0.89 | 8.00 | 10.00 | 8.31 | 0.74 | 8.00 | 10.00 | 8.29 | 0.71 | 8.00 | 10.00 | 8.46 | 0.85 | 8.00 | 10.00 | - | - |
| NEE | 6.00 | 0.32 | 5.00 | 7.00 | 6.09 | 0.47 | 5.00 | 7.00 | 6.17 | 0.38 | 6.00 | 7.00 | 6.15 | 0.39 | 5.00 | 7.00 | - | - |
Table 1. Descriptive statistics for the 27 variables on H. vittatus.
For these 5 characters, Mann-Whitney U Tests were done for examining if there is any significance difference between sexes and between populations. The outcomes of this test showed that these characters are not candidate characters for sex or geography differentiation (p>0.05) (Table 2).
| Test | MSOL | NSL | NIL | RVS | NEE |
|---|---|---|---|---|---|
| Grouping variable: Sex | |||||
| Mann-Whitney U | 5243 | 5187.5 | 5127.5 | 5039 | 5255.5 |
| Asymp. Sig (2-tailed) | 0.949 | 0.601 | 0.39 | 0.434 | 0.958 |
| Grouping variables: Geography | |||||
| Mann-Whitney U | 4102 | 3953 | 3981 | 4092 | 3731 |
| Asymp. Sig (2-tailed) | 0.987 | 0.269 | 0.387 | 0.951 | 0.129 |
Table 2. Results of Mann-Whitney U Tests. (the upper half shows the sex comparisons, and the lower half shows the geographic comparisons. Each one ignores the other in group analysis).
Only 9 of 27 morphometric measurements showed statistically significant patterns according to results of ANOVA tests. These characters are SVL, HL, FLL, HLL, NED, IPW, PW, NDS and NVS. After that applied post hoc test, Tukey’s HSD demonstrated that differences observed in NDS and NVS occurred only between Anatolian males and Arabian females. Therefore, these characters were also excluded from analysis (Table 3). According to these results, only sexually dimorphic characters were seen as follows: i) SVL between Arabian females (mean ± SD=76.41 ± 9.12 mm) and Arabian males (71.02 ± 6.96 mm), ii) HLL between Arabian females (25.63 ± 2.38 mm) and Arabian males (24.94 ± 2.30 mm). In this regard, only geographic variation patterns were seen as follows:
| Multiple Comparisons | SVL | FLL | HLL | NED | FIL | IPW | PW | NDS | NVS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Arabian Male | Anatolian Male | 0.919 | 0.185 | 0.177 | 0.047 | 1 | 0.043 | 0 | 0.209 | 0.157 |
| Arabian Female | 0.001 | 0.299 | 0.005 | 0.901 | 0.674 | 0.76 | 0.955 | 0.529 | 0.795 | |
| Anatolian Male | Anatolian Female | 0.13 | 0.69 | 0.414 | 0.783 | 0.542 | 0.688 | 0.767 | 0.615 | 0.394 |
| Arabian Female | Anatolian Female | 0.8 | 0.081 | 0.243 | 0.065 | 0.037 | 0.54 | 0.007 | 0.263 | 0.649 |
Table 3. Results of Tukey HSD Post Hoc tests after ANOVA.
i. NED and IPW for males from Arabian Plate (3.37 ± 0.35 mm and 1.88 ± 0.22 respectively) and Anatolia (3.11 ± 0.34 mm and 1.73 ± 0.19 mm respectively),
ii. FIL for females (Arabian 4.62 ± 0.52 mm, Anatolian 4.34 ± 0.53 mm). Lastly PW showed sexual dimorphism and geographic variation patterns in both populations (Arabian ♀: 3.38 ± 0.36 mm, Arabian ♂: 3.41 ± 0.34 mm; Anatolian ♀: 3.05 ± 0.27 mm, Anatolian ♂: 3.14 ± 0.40 mm) (p<0.05).
PCA results from the remaining 7 morphometric variables revealed that the first three principal components 60.91%, 13.60% and 9.25% of the total variation respectively (Fig. 3) (Table 4). Of these, the first principal component (PC1) was strongly and negatively correlated with SVL, FLL and HLL as almost the same impact. PC2 was strongly and positively correlated with FIL and negatively correlated with IPW. Lastly PC3 was strongly and positively correlated with IPW.
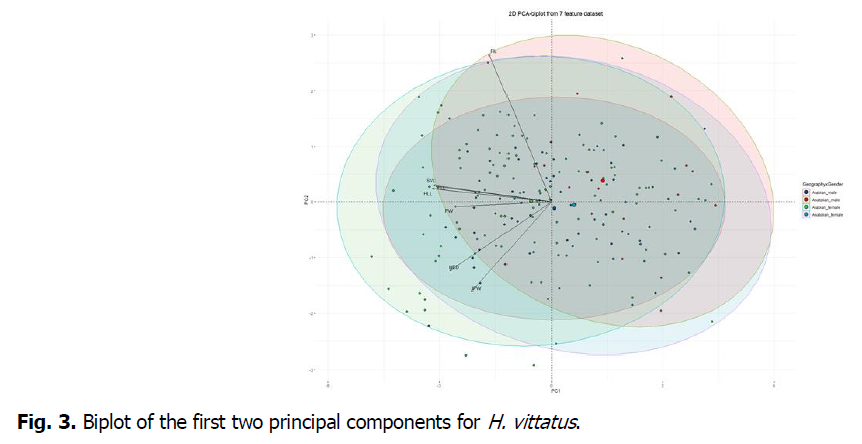
Fig 3. Biplot of the first two principal components for H. vittatus.
| Components | PC1 | PC2 | PC3 |
|---|---|---|---|
| SVL | -0.441 | 0.09 | -.0.283 |
| FLL | -0.431 | 0.083 | -0.261 |
| HLL | -0.446 | 0.071 | -0.252 |
| NED | -.0.380 | -0.365 | -0.139 |
| FIL | -0.232 | 0.786 | 0.456 |
| IPW | -0.298 | -0.477 | 0.709 |
| PW | -0.363 | -0.024 | 0.236 |
| Eigenvalue | 4.264 | 0.952 | 0.647 |
| Prop. Of Variance/Trace | 0.6091 | 0.1361 | 0.0925 |
| Cum. Proportion | 0.6091 | 0.7452 | 0.8377 |
Table 4. Factor Loadings for the First Three Principal Components of variation (PC1, PC2 and PC3) of Seven Morphological Characters in H. vittatus.
Discussion
Despite its wide distribution range along Anatolia, Middle East and North Africa, H. vittatus remains relatively poorly studied in Anatolia. There have been several studies on its distribution (Fattahi et al., 2013), local sexual dimorphism (Rastegar-Pouyani and Fattahi, 2015) with reproductive systems given with (Nassar et al., 2013; Nassar and Hraoui-Bloquet, 2014) and geographic variation (Fattahi et al., 2014b, Rastegar-Pouyani et al., 2021). However, the study on the phylogeny of genus Heremites provided us a sight that, there might be a difference between these “Anatolian” and “Arabian” populations (Güçlü et al., 2014; Karin et al., 2016).
We found that Arabian females’ SVL are higher than Arabian males, but no difference for Anatolian populations in this trait (Table 1). These results are compatible with Nassar et al (2013) study in Lebanon and Rastegar-Pouyani and Fattahi (2015) study in Zagros Mountains. Because Zagros and Assyrian Sutures comprises the Arabian Platform as the same continental shift within the same topography of Anatolian’s Arabian plate (Okay, 2008). On the other hand, although Anatolian females’ SVL are higher than Anatolian males’, this pattern did not reflect to statistics (Table 1). However, it is a well-known phenomenon since (Darwin, 1871) that natural selection should support large female size, if this trait has a positive correlation with fecundity within a population for viviparous species (Cox et al., 2003; Becker and Paulissen 2012; Karamiani et al., 2018). Additionally the presence of ovoviviparity in H. vittatus, it might be claimed that due to an energy investment in producing offsprings, females can produce a longer and wider trunk as a result of fecundity selection (Rastegar-Pouyani and Fattahi, 2015).
Although SVL decreases with its correlation of altitude throughout the Anatolian Peninsula (Yıldırım et al., 2021), no information is available for other morphometric parameters of this skink species in terms of elevation up to now. However, it is clear that there is no sexual differentiation pattern between male and female specimens was observed among Anatolian populations. In contrast to Anatolian populations, the sexual dimorphism was observed with two characters (SVL and HLL) in Arabian populations. This dimorphic pattern also demonstrated that, female-biased differences in head size and limb characteristics in both populations might be related to bite force and chasing the prey activities. This might be possible due to females eat larger prey than males and their energy allocation for reproduction is higher than males (Wiederhecker et al., 2005). However it is hypothetical because studies on comparisons of bite force or diet composition and prey size between H. vittatus females and males are still lacking (Rastegar-Pouyani and Fattahi, 2015).
On the other hand, our results for NED, IPW and PW differences between males from both populations and FIL, PW differences between females showed us the geographic differentiation patterns. These results are also compatible to the phylogenetic study on this species, which shows this little genetic variation (Güçlü et al., 2014). This kind of intraspecific variation pattern can be easily demonstrated in reptiles. Geography could be a determinative factor for intraspecific variations, however the challenging factors may decide the level of differentiation whether it is high or not (Kaliontzopoulou et al., 2010). In our case, Anatolian part of the Arabian plate has slight effect on shaping the morphology of this skink species. However, environmental factors, such as temperature could affect the invertebrate community dynamics (Robinson et al., 2018) in this region more than main Anatolian peninsula. Because invertebrate animals are the main nutrition resources of this species.
This study demonstrated sexual dimorphism and geographic variation patterns in H. vittatus populations. While Arabian populations’ males and females are slightly different from each other, this overall pattern could not be observed in Anatolian populations. As a conclusion, these results provide a basis for further comparative studies on the changes in shape and body size that are not only related to environmental dynamics, but also behavioral traits, like bite force or prey size preferences.
References
Ashton, K.G., Burke, R.L., Layne, J.N. (2007). Geographic variation in body and clutch size of gopher tortoises. Copeia, 2:355-363.
Bager, A., Lucas, P.S., Costa, A., Lima, J.C.S., Silveira, M.L. (2016). Geographical variation in the morphology and sexual dimorphism of Acanthochelys spixii (Testudines, Chelidae) in Brazil. Tropical Zoology, 29:73-86.
Baran, İ. (1977). Türkiye’de Scincidae familyası türlerinin taksonomisi. Doğa Bilim Dergisi, 1:217-223 (in Turkish).
Basoğlu, M., Baran, I. (1977). Türkiye Sürüngenleri, Kısım I, Kaplumbağa ve Kertenkeleler. Ege Üniversitesi Kitaplar Serisi, 76:1-219 (in Turkish).
Becker, B.M., Paulissen, M.A. (2012). Sexual dimorphism in head size in the little brown skink (Scincella lateralis). Herpetological Conservation and Biology, 7:109-114.
Budak, A. (1973). Türkiye’de Mabuya vittata (Scincidae, Lacertilia)‘nın bireysel ve coğrafik variasyonu üzerinde çalışmalar. Ege Üniv Fen Fakültesi İlmi Raporlar Serisi, 162:1-25 (in Turkish).
Cox, R.M., Skelly, S.L., John‐Alder, H.B. (2003). A comparative test of adaptive hypotheses for sexual size dimorphism in lizards. Evolution, 57:1653-1669.
Cruz-Elizalde, R., Ramírez-Bautista, A., Lozano, A. (2017). Sexual size dimorphism among populations of the rose-bellied lizard Sceloporus variabilis (Squamata: Phrynosomatidae) from high and low elevations in Mexico. Herpetological Journal, 27:252:257.
Cıplak, B. (2004). Biogeography of Anatolia: the marker group Orthoptera. Memorie della Societa Entomologica Italiana, 82:357-372.
Darwin, C. (1871). The descent of man, and selection in relation to sex. Princeton: Princeton UP.
DeGregorio, B.A., Blouin-Demers, G., Carfagno, G.L., Gibbons, J.W., Mullin, S.J., Sperry, J.H., Willson, J.D., Wray, K., Weatherhead, P.J. (2018). Geographic variation in body size and sexual size dimorphism of North American Ratsnakes (Pantherophis spp. sl). Canadian Journal of Zoology, 96:1196-1202.
Fattahi, R., Ficetola, G.F., Rastegar-Pouyani, N., Avcı, A., Kumlutaş, Y., Ilgaz, C., Hosseinian Yousefkhani, S.S. (2014a). Modelling the potential distribution of the Bridled Skink, Trachylepis vittata (Olivier, 1804), in the Middle East. Zoology in the Middle East, 60:208-216.
Fattahi, R., Rastegar-Pouyani, N., Seymareh, H.A. (2014b). Notes taxonomy, distribution, geographic variation and natural history in Trachylepis vittata (Olivier, 1804) in the Zagros Mountains, Western Iran. Russian Journal of Herpetology, 21:179-186.
Fattahi, R., Yousefkhani, S.S.H., Ilgaz, C., Kumlutas, Y., Avci, A., Rastegar-Pouyani, N. (2013). Distribution of Trachylepis vittata (Olivier, 1804) in Middle East and North Africa. WebPub, 1:55-57.
Greer, A.E. (1977). The systematics and evolutionary relationships of the scincid lizard genus Lygosoma. Journal of Natural History, 11: 515-540.
Güçlü, Ö., Candan, K., Kankiliç, T., Kumlutaş, Y., Durmuş, S.H., Poulakakis, N., Ilgaz, C. (2014). Phylogeny of Trachylepis sp.(Reptilia) from Turkey inferred from mtDNA sequences. Mitochondrial DNA, 25:456-463.
Hendry, A.P. (2020). Eco-evolutionary dynamics. Princeton University Press.
Karamiani, R., Rastegar-Pouyani, N., Rastegar-Pouyani, E. (2018). Sexual dimorphism in the asian snake-eyed skink, Ablepharus pannonicus (Fitzinger, 1823 (Sauria): Scincidae) from Iran. Russian Journal of Herpetology, 25:1-5.
Kaliontzopoulou, A., Carretero, M.A., Sillero, N. (2010). Geographic patterns of morphological variation in the lizard Podarcis carbonelli, a species with fragmented distribution. The Herpetological Journal, 20:41-50.
Karin, B.R., Metallinou, M., Weinell, J.L., Jackman, T.R., Bauer, A.M. (2016). Resolving the higher-order phylogenetic relationships of the circumtropical Mabuya group (Squamata: Scincidae): An out-of-Asia diversification. Molecular Phylogenetics and Evolution, 102:220-232.
Kroeker, K.J., Sanford, E., Rose, J.M., Blanchette, C.A., Chan, F., Chavez, F.P., Gaylord, B., Helmuth, B., Hill, T.M., Hofmann, G.E., McManus, M.A., Menge, B.A., Nielsen, K..J., Raimondi, P.T., Russell, A.D., Washburn, L. (2016). Interacting environmental mosaics drive geographic variation in mussel performance and predation vulnerability. Ecology Letters, 19:771-779.
Kumlutas, Y., Candan, K., Ilgaz. C. (2015). A new locality record of Trachylepis vittata (Olivier, 1804) (Reptilia: Scincidae) in Northeastern Anatolia, Turkey. Russian Journal of Herpetology, 22:310-314.
Kwiatkowski, M.A., Sullivan, B.K. (2002). Geographic variation in sexual selection among populations of an iguanid lizard, Sauromalus obesus (= ater). Evolution, 56:2039-2051.
Mausfeld, P., Vences, M., Schmitz, A., Veith, M. (2000). First data on the molecular phylogeography of scincid lizards of the genus Mabuya. Molecular Phylogenetics and Evolution, 17:11-14.
Mayr, E. (1956). Geographical character gradients and climatic adaptation. Evolution, 10:105-108.
Mayr, E. (1982). The growth of biological thought: Diversity, Evolution and Inheritance. Harvard University Press.
Mulder, J. (1995). Herpetological observations in Turkey (1987-1995). Deinsea, 2:51-66.
Nassar, F., Challita, M., Sadek, R., Hraoui-Bloquet, S. (2013). Sexual dimorphism and female reproductive cycle in the scincid lizard Trachylepis vittata (Olivier, 1804) in Lebanon (Reptilia: Scincidae). Zoology in the Middle East, 59:297-301.
Nassar, F., Hraoui-Bloquet, S. (2014). Distribution and male reproductive system of the Bridled Mabuya, Trachylepis vittata (Olivier, 1804) (Reptilia: Scincidae), in Lebanon. Zoology in the Middle East, 60:314-319.
Okay, A.I. (2008). Geology of Turkey: a synopsis. Anschnitt 21:19-42.
Ozdemir, A., Durmus, S.H., Kete, R., Yılmaz, I. (2001). Hatay ve Gaziantep Mabuya vittata Olivier 1804 (Lacertilia: Scincidae) Ornekleri Uzerinde Bir Arastırma. Anadolu Universitesi Bilim ve Teknoloji Dergisi, 2:271-275 (in Turkish).
Pianka, E.R., Vitt, L.J. (2003). Lizards: windows to the evolution of diversity. University of California Press.
R Core Team. (2018). R: A language and environment for statistical computing, R foundation for statistical computing. Vienna: R Core Team.
Ramiro, C.N., Recoder, R.S., Rodrigues, M.T. (2019). Geographic variation in the morphology of the sand-dwelling lizard Nothobachia ablephara (Squamata: Gymnophthalmidae). Phyllomedusa: Journal of Herpetology, 18:195-207.
Rastegar-Pouyani, N., Fattahi, R. (2015). Sexual dimorphism in Trachylepis vittata (Olivier, 1804) (Sauria: Scincidae) in the Zagros Mountains, Western Iran. Turkish Journal of Zoology, 39:59-65.
Rastegar-Pouyani, N., Kumlutas, Y., Avci, A., Candan, K., Ilgaz, C., Fattahi, R., Yousefkhani, S.S.H. (2021). Phenotypic variation in Heremites vittatus (Olivier, 1804) (Sauria: Scincidae) from Iran and Turkey. Zootaxa, 5004:181-192.
Rivera, G. (2008). Ecomorphological variation in shell shape of the freshwater turtle Pseudemys concinna inhabiting different aquatic flow regimes. Integrative and Comparative Biology, 48:769-787.
Robinson, S.I., McLaughlin, Ó.B., Marteinsdóttir, B., O'Gorman, E.J. (2018). Soil temperature effects on the structure and diversity of plant and invertebrate communities in a natural warming experiment. Journal of Animal Ecology, 87:634-646.
Roitberg, E.S., Eplanova, G.V., Kotenko, T.I., Amat, F., Carretero, M.A., Kuranova, V.N., Bulakhova, N.A., Zinenko, O.I., Yakovlev, V.A. (2015). Geographic variation of life‐history traits in the sand lizard, Lacerta agilis: testing Darwin’s fecundity‐advantage hypothesis. Journal of Evolutionary Biology, 28:613-629.
Şenkul, C., Dogan, U. (2013). Vegetation and climate of Anatolia and adjacent regions during the Last Glacial period. Quaternary International, 302:110-122.
Slatkin, M. (1987). Gene flow and the geographic structure of natural populations. Science, 236:787-792.
Stoner, G., Breden, F. (1988). Phenotypic differentiation in female preference related to geographic variation in male predation risk in the Trinidad guppy (Poecilia reticulata). Behavioral Ecology and Sociobiology, 22:285-291.
Uetz, P., Freed, P., Hošek, J. (2022). The Reptile Database.
Wallace, B.P., Saba, V.S. (2009). Environmental and anthropogenic impacts on intra specific variation in leatherback turtles: opportunities for targeted research and conservation. Endangered Species Research, 7:11-21.
Wiederhecker, H., Pinto, A., Colli, G. (2005). Sexual dimorphism in the Neotropical lizard, Tropidurus torquatus (Squamata, Tropiduridae). Amphibia-Reptilia, 26:127-137.
Yıldırım, E., Kumlutas, Y., Candan, K., Ilgaz, C. (2021). The study on the relationships between the age structure and body size of the bridled skink, Heremites vittatus, (Oliver, 1804) from Different Altitudes in Turkey. Journal of the Institute of Science and Technology, 11:906-915.
Zar, J.H. (2010). Biostatistical analysis. 5th edition, Prentice-Hall, New Jersey, p:944.
Author Info
M.K. Sahin1*, E.Y. Caynak2,3, Y. Kumlutas2,3, K. Candan2,3 and C. Ilgaz2,32Department of Biology, Faculty of Science, Dokuz Eylul University, Buca, Izmir, Turkey
3Research and Application Center for Fauna and Flora, Dokuz Eylul University, 35610, Buca, Izmir, Turkey
Citation: Sahin, M.K., Caynak, E.Y., Kumluta, Y., Candan, K., Ilgaz, C. (2022). Sexual dimorphism and geographic variation in the bridled skink, Heremites vittatus (Oliver, 1804), (Sauria: Scincidae) in Anatolian Peninsula. Ukrainian Journal of Ecology. 12:1-7.
Received: 23-Feb-2022, Manuscript No. uje-22-55340; , Pre QC No. P-55340; Editor assigned: 26-Feb-2022, Pre QC No. P-55340; Reviewed: 10-Mar-2022, QC No. Q-55340; Revised: 19-Mar-2022, Manuscript No. R-55340; Published: 29-Mar-2022, DOI: 10.15421/2022_358
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
