Research Article - (2022) Volume 12, Issue 3
Study of infestation and harmfulness degree of weeds on fodder barley (Hordeum vulgare L.) in an arid region (Laghouat-Algeria)
M. Hattab*Abstract
37 floristic surveys were carried out in an arid region with the aim to study the degree of infestation and harmfulness of weeds on fodder barley carried out under irrigation. 36 weed species have been inventoried, they belong to 16 families, the most dominant of which are the Brassicaceae (22.2%), the Asteraceae (19.4%) and the Poaceae (13.8%). Dicotyledons are in the majority (86.2%) and Therophytes (67%) characterize this flora. The study of the Partial Harmfulness Index (PHI) revealed the existence of 19 species which are considered to be potentially harmful to fodder barley, 12 of which have a PHI greater than 1000. Thus, the perfect knowledge of the biology of weeds that potentially harmful and infesting could help to judiciously choose the adequate weed control interventions in order to optimize the profitability of the applications.
Keywords
Laghouat, Weeds, Fodder barley, Infestation, Harmfulness.
Introduction
In Algeria, fodder resources intended for feeding livestock are limited and are largely provided by rangelands (steppe and forest), fallow land, natural grasslands, and cereal by-products (cereal stubble, straw) (Hamrit, 1995; Abdelguerfi et al., 2008; Issolah, 2008).
Fodder crops occupy a marginal place in plant production (Abdelguerfi et al., 2008). In addition to the small area reserved for these crops, diversity of species is very limited and crops of vetch-oats, barley and oats, intended for production hay, are the main crops in Algeria.
Fodder and pastoral production, which seems limited in Algeria, often represents a brake on the development of livestock farming. Each year, large quantities of fodder are imported to cover the feed deficit of livestock (Issolah, 2008; Hadbaoui et al., 2020).
In order to satisfy food needs of the livestock in Algeria, various solutions can be envisaged such as development of fodder crops although they are dependent on the control of crop management. The control of weeds is an essential operation for the successful establishment of a fodder crop and limiting their impact on quantitative and qualitative yield. Chemical weeding is little used in Algeria, so the control of weeds in fodder crops requires knowledge of the most harmful species. This study, conducted in a steppe region with an arid climate, is therefore designed to analyze the weed flora that accompanies fodder barley grown under irrigated conditions.
Materials and Methods
Study region
Fodder barley is widely cultivated in Ben Nacer Ben Chohra, which is a region belonging to the Laghouat province (Fig. 1). This steppe region is characterized by an arid climate where sheep farming is practiced on a large scale.
Fig 1: Location of the study region (Daoudi et al., 2021).
Plant material
Barley (Hordeum vulgare L.) is a straw cereal belonging to the Poaceae family, it is cultivated in the study region for animal feed. In this region, barley is the main fodder crop with alfalfa. It is irrigated using groundwater mobilized by a sprinkler system. Barley is used by breeders either as grain or pasture or cut and distributed fresh to feed their livestock. No weeding action is practiced on this crop, hence the importance of measuring the consequences by evaluating the degree of infestation and harmfulness of weeds.
Cultivation of fodder barley in the study region
Breeders in this region use fodder crops to fill the food deficit of their livestock. With the regression of steppe rangelands, breeders have been forced to look for additional fodder resources to feed their herds which are constantly increasing in number. This is why some breeders have become mixed breeders and cultivate fodder (Hadbaoui et al., 2020).
Realization of floristic surveys
floristic surveys were carried out on barley plots chosen at random. Each survey covers approximately an area of 2500 m2. 37 floristic surveys were carried out mainly at the flowering stage of weeds, from the end of February until the beginning of April 2021. The fact that floral induction depends in part on ambient temperature and sunshine in spring, the flowering of plants in Algeria is generally advanced one to two weeks each time we move towards the south of the country due to the high temperature and sunshine that characterize the climate of arid and Saharan regions.
Floristic survey technique used is that of the «around field», which is the most exhaustive (Maillet, 1981; Chicouene, 1999). This consists of positioning in the field by checking that there is no ecologically particular zone (rocky outcrop, wetter zone, change in the nature of the soil, etc.) which could influence the selection of species. Once in the plot, the assessor notes all the species present around him, then he gradually moves in all directions, noting the new species until he no longer sees any new species. Once all the species have been inventoried, the assessor makes a second quick turn to assign an abundance-dominance value to each of the species. The abundance-dominance index is assessed over the entire area in which the inventory was made. This is why the assessor first makes an exhaustive inventory of the species (which already gives a first idea of their distribution and their abundance-dominance), and then he notes the abundance-dominance after having had this global vision of the area (Le Bourgeois, 1993).
Abundance-dominance index does not need to be very precise, the important thing is to be able to compare the species with each other, the dominant ones with the minor ones by referring to the recovery rate of each species. When studying a particular species, we choose the abundance-dominance index rating scale that is most representative of the species’ behavior according to its biological type. But in our case, we are studying grass cover where species have different biological types, which is why we considered it sufficient to choose the Marnotte (1984) rating scale, which is from 1 to 9 levels, to compare the species (Table 1). This scale gives a closer idea of the recovery rate of the species in the field than that of Braun-Blanquet (1952) at 6 levels.
| Abundance-dominance index | % of recovery | Recovery |
|---|---|---|
| 1 | 1 | Species present, but rare |
| 2 | 7 | Less than 1 individual per m² |
| 3 | 15 | At least 1 individual per m² |
| 4 | 30 | 30% of recovery |
| 5 | 50 | 50% of recovery |
| 6 | 70 | 70% of recovery |
| 7 | 85 | High recovery |
| 8 | 93 | Very little visible soil |
| 9 | 100 | Total recovery |
Table 1. Recovery scale proposed by Marnotte (1984).
The following identification keys were used to identify the inventoried species: Quezel and Santa (1962), Quezel and Santa (1963), Ozenda (1991), ACTA (1997), Chehma (2006), and the application PlantNet.
Qualitative and quantitative analysis
The data collected were analyzed using two floristic approaches: qualitative and quantitative.
Qualitative floristic analysis has identified the composition of weed flora of fodder barley in our study region, as well as the biological type of each species.
Quantitative floristic analysis has defined the agronomic importance of the various inventoried species by determining infestation degree and partial harmfulness index (PHI) of each species.
Abundance and frequency are the most effective parameters for measuring crop infestation by weeds (Barralis, 1976; Bouhache and Boulet, 1984; Traore and Maillet, 1998). The infestation diagram is represented by positioning of the species on a graph where the relative frequency of the species in a survey set is plotted on the abscissa and their abundance on the ordinate. This diagram distinguiches groups of species according to their infestation degree, and thus their agronomic importance (Traore and Maillet, 1998). Abundance index used is the average abundance-dominance index (calculated in relation to the number of surveys in which the species is present) which gives the species a similar weight at the level of the graph and makes it possible to easily delimit the sectors corresponding to the different groups (Le Bourgeois, 1993).
Evaluation of the harmfulness exerted by weeds on fodder barley was calculated by taking into account, for each species, the abundance-dominance index, the absolute frequency and the biological type. These parameters made it possible to assign each species a Partiel Harmfulness Index (PHI) (Bouhache and Boulet, 1984). This index is obtained by transforming the abundance-dominance index into an average recovery percentage. Thus, the partial harmfulness index was determined according to the following formula:
PHI=(Sum of average recoveries of the species/Absolute frequency) × 100
(Bouhache and Boulet, 1984; Zidane et al., 2010; Ka et al., 2020; Melakhessou et al., 2020). This index is underlined in Table 3 when the species is a perennial, and will be followed by the relative frequency of each species. Combination of the total abundance and the relative frequency of species makes it possible to identify the potential risks of harmfulness on a regional scale (Barralis, 1976; Bouhache and Boulet, 1984). Minor species with a relative frequency of less than 20% are not taken into account for the calculation of this index.
Results and Discussion
Floristic composition
The 37 surveys carried out made it possible to inventory 36 species of weeds belonging to 16 families (Table 2). This number of species is very low compared to other studies (Tanji and Ait Lhaj, 2010; Zidane et al., 2010; Chafik et al., 2012; Hannachi and Fenni, 2013; Karkour and Fenni, 2016; Melakhessou et al., 2020). The few weeds can be explained by the cultivation of perennial alfalfa as the previous crop of fodder barley in this region. This is what is actually observed by the presence of regrowth of this legume in many of the surveys carried out (Table 2). Perennial alfalfa can smother weeds and reduce their numbers remarkably (Bonte, 2010; Le Chatelier et al., 2016).
| Species | EPPO code | Family | Class | Absolute frequency | Relative frequency | Biological type |
|---|---|---|---|---|---|---|
| Aethusa cynapium L. | AETCY | Apiaceae | D | 12 | 0,32 | Th |
| Arabidopsis thaliana (L.) Heynh. | ARBTH | Brassicaceae | D | 08 | 0,21 | Th |
| Avena fatua L. | AVEFA | Poaceae | M | 05 | 0,13 | Th |
| Beta vulgaris L. | BEAVX | Amaranthaceae | D | 05 | 0,13 | He |
| Bromus sterilis L. | BROST | Poaceae | M | 02 | 0,05 | Th |
| Buglossoides arvensis (L.) I.M. Johnst | LITAR | Boraginaceae | D | 03 | 0,08 | Th |
| Calendula arvensis L. | CLDAR | Asteraceae | D | 19 | 0,51 | Th |
| Centaurea calcitrapa L. | CENCA | Asteraceae | D | 33 | 0,89 | He |
| Chenopodium album L. | CHEAL | Amaranthaceae | D | 04 | 0,10 | Th |
| Convolvulus arvensis L. | CONAR | Convolvulaceae | D | 25 | 0,67 | He |
| Cynodon dactylon (L.) Pers. | CYNDA | Poaceae | M | 02 | 0,05 | Ge |
| Diplotaxis erucoides (L.) DC. | DIPER | Brassicaceae | D | 03 | 0,08 | Th |
| Erodium laciniatum (Cav.) Willd. | EROLA | Geraniaceae | D | 01 | 0,02 | Th |
| Eruca vesicaria (L.) Cav. | ERUVV | Brassicaceae | D | 15 | 0,40 | Th |
| Euphorbia helioscopia L. | EPHHE | Euphorbiaceae | D | 02 | 0,05 | Th |
| Fumaria parviflora Lam. | FUMPA | Papaveraceae | D | 24 | 0,64 | Th |
| Galium aparine L. | GALAP | Rubiaceae | D | 18 | 0,48 | Th |
| Glebionis segetum (L.) Fourr. | CHYSE | Asteraceae | D | 04 | 0,10 | Th |
| Hordeum murinum L. | HORMU | Poaceae | M | 01 | 0,02 | Th |
| Lepidium draba L. | CADDR | Brassicaceae | D | 29 | 0,78 | He |
| Malva parviflora L. | MALPA | Malvaceae | D | 31 | 0,83 | Th |
| Medicago sativa L. | MEDSA | Fabaceae | D | 23 | 0,62 | He |
| Moricandia suffruticosa (Desf.) Coss. & Durieu | MOCSU | Brassicaceae | D | 01 | 0,02 | He |
| Onopordum acanthium L. | ONRAC | Asteraceae | D | 12 | 0,32 | He |
| Papaver hybridum L. | PAPHY | Papaveraceae | D | 10 | 0,27 | Th |
| Peganum harmala L. | PEGHA | Nitrariaceae | D | 01 | 0,02 | Ch |
| Phalaris minor Retz. | PHAMI | Poaceae | M | 03 | 0,08 | Th |
| Polygonum aviculare L. | POLAV | Polygonaceae | D | 06 | 0,16 | Th |
| Reichardia picroides (L.) Roth | REIPI | Asteraceae | D | 01 | 0,02 | He |
| Reseda lutea L. | RESLU | Resedaceae | D | 01 | 0,02 | He |
| Silybum marianum (L.) Gaertn. | SLYMA | Asteraceae | D | 32 | 0,86 | He |
| Sinapis alba L. | SINAL | Brassicaceae | D | 29 | 0,78 | Th |
| Sinapis arvensis L. | SINAR | Brassicaceae | D | 34 | 0,91 | Th |
| Sisymbrium irio L. | SSYIR | Brassicaceae | D | 17 | 0,45 | Th |
| Sonchus oleraceus L. | SONOL | Asteraceae | D | 15 | 0,40 | Th |
| Vicia sativa L. | VICSA | Fabaceae | D | 20 | 0,54 | Th |
Table 2. Botanical aspects and biological types of inventoried species.
Among the species of weeds identified, Dicotyledons are the majority with 31 species, or 86.2%. While the Monocotyledons are represented by only 5 species, or 13.8%.
Of all the families identified, 3 clearly dominate the weed flora of fodder barley (Fig. 2): Brassicaceae (22.2%), Asteraceae (19.4%), and Poaceae (13.8%). The other families each do not exceed 5.5%.
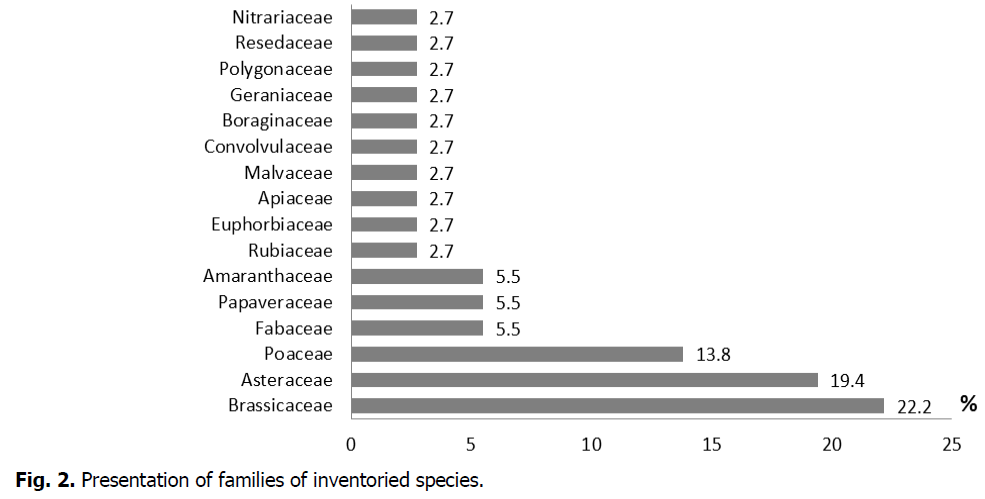
Fig 2: Presentation of families of inventoried species.
Biological spectrum
As far as the biological spectrum, the Raunkiaer (1905) classification was used, which is based on the position of permanent buds in relation to the soil surface during the vegetative rest period.
According to Fig. 3, weeds in fodder barley fields in our study region belong to 4 biological types which are in decreasing order: Therophytes (67%), Hemicryptophytes (28%), Geophytes (2.5%), and Chamaephytes (2.5%).
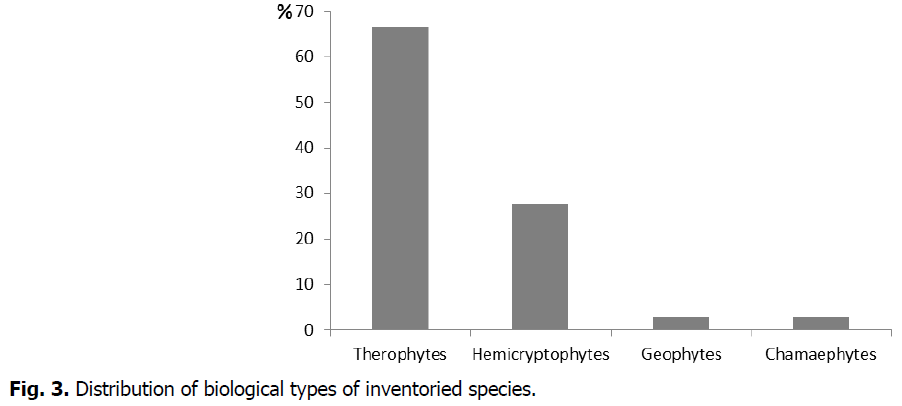
Fig 3: Distribution of biological types of inventoried species.
This high rate of Therophytes is similar to that found by other authors (Zidane et al., 2010; Chafik et al., 2012; Hannachi and Fenni, 2013; Karkour and Fenni, 2016; Melakhessou et al., 2020). Indeed, Taleb et al. (1997) reported that the cultivation techniques used facilitate their development compared to other biological types. Maillet (1981), also indicated that repeated tillage tends to eliminate perennial species in favor of Therophytes.
Infestation degree
Infestation diagram produced from the 37 surveys revealed 9 groups of species (Fig. 4). These different groups reflect their infestation potential, and therefore their agronomic importance.
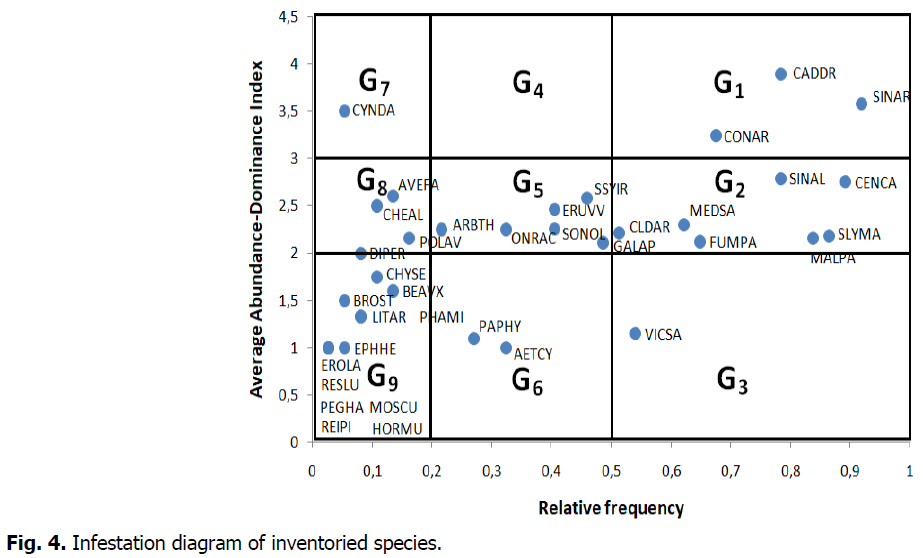
Fig 4: Infestation diagram of inventoried species.
General major weeds (G1): They are the most infesting species. This group is represented by 3 species (Sinapis arvensis, Lepidium draba, and Convolvulus arvensis) which were encountered in more than 50% of the surveys with high recovery (average AD ≥ 3). The importance of these three species is due to the precocity of their emergence, or to their high seed production, or to their vegetative propagation (Warwick et al., 2000; Bond et al., 2007; Francis and Warwick, 2007; Jacobs, 2007; Guerin, 2016; Sosnoskie et al., 2020), or to their adaptation to cultural practices. These species can also cause food poisoning in livestock (Mulligan and Munro, 1990; Allison et al., 2016; Graves-Medley and Mangold, 2018; Sosnoskie et al., 2020; Keywanloo et al., 2021).
General potential weeds (G2): They are ubiquitous species found in the majority of plots (Fr>50%), but their infestation is less or very localized when it is high (2 ≤ average AD<3). This group is represented by 7 species (Centaurea calcitrapa, Sinapis alba, Silybum marianum, Malva parviflora, Fumaria parviflora, Calendula arvensis). This group of species can easily adapt to environments disturbed by cultural interventions (Fenni, 2003). Indeed, these types of species can cause significant harm to the crop of fodder barley. In addition, Centaurea calcitrapa, Silybum marianum, Malva parviflora, can cause poisoning and intoxication to livestock, especially sheep and cattle (Main and Butler, 2006; Agaie et al., 2007; Mohammedi et al., 2014; Allison et al., 2016).
General weeds (G3): This group is made up of common species but with generally low recovery (average AD<2). This group is formed by a single species: Vicia sativa. This plant is apparently harmless to fodder barley yield.
Regional potential weeds (G5): This group is made up of species with a medium relative frequency and medium recovery. This group is represented by 6 species (Galium aparine, Sisymbrium irio, Eruca vesicaria, Sonchus oleraceus, Onopordum acanthium, Arabidopsis thaliana). These species can compete with fodder barley on water and fertilizing elements but less so than in groups 1 and 2. Both Sisymbrium irio and Sonchus oleraceus can cause poisoning and intoxication in livestock (Belanger, 2020; Hall et al., 2020).
Regional weeds (G6): This group includes species with medium ecological amplitude (20% ≤ Fr<50%) and low recovery (average AD<2). This group is represented by only 2 species (Aethusa cynapium and Papaver hybridum). Apparently, these two species do not pose any particular problems for fodder barley yield. However, Aethusa cynapium can cause poisoning in livestock (ANES, 2021).
Local major weeds (G7): this group represents infrequent species but their recovery is high (average AD ≥ 3). This group contains a single species (Cynodon dactylon) which, when abundant, can constitute, on a local scale, an important agronomic constraint because of the difficulty that farmers find in controlling it. It is a species that propagates by seed and especially by vegetative means (Di Tomaso et al., 2013).
Local potential weeds (G8): These species are very rarely encountered in the plots, but with medium recovery. This group is made up of 4 species (Avena fatua, Chenopodium album, Polygonum aviculare, and Diplotaxis erucoides). These species do not greatly interfere with growth and development of fodder barley. Chenopodium album and Polygonum aviculare can cause poisoning and intoxication in livestock (Mulligan and Munro, 1990; Allison et al., 2016; Stubbendieck et al., 2018; Belanger, 2020).
Minor weeds (G9): This group contains species with low frequency and low recovery. 12 species represent this group, and do not pose any particular problems for fodder barley crops.
Group 4 which corresponds to the regional major weeds has no representative in this flora.
Partiel Harmfulness Index (PHI)
Classification of weeds according to their partiel harmfulness index and their relative frequency revealed the existence of 19 species considered as potentially harmful to fodder barley in Ben Nacer Ben Chohra region (Table 3).
| Species | PHI | Relative frequency (%) |
|---|---|---|
| Group 01 : PHI ≥ 1000 | ||
| Lepidium draba L. | 3013 | 78,37 |
| Sinapis arvensis L. | 2715 | 91,89 |
| Convolvulus arvensis L. | 2180 | 67,56 |
| Centaurea calcitrapa L. | 1888 | 89,18 |
| Sinapis alba L. | 1710 | 78,37 |
| Onopordum acanthium L. | 1475 | 32,43 |
| Sisymbrium irio L. | 1388 | 45,94 |
| Eruca vesicaria (L.) Cav. | 1340 | 40,54 |
| Medicago sativa L. | 1140 | 62,16 |
| Sonchus oleraceus L. | 1073 | 40,54 |
| Silybum marianum (L.) Gaertn. | 1028 | 86,48 |
| Galium aparine L. | 1028 | 48,64 |
| Group 02 : 500<PHI<1000 | ||
| Calendula arvensis L. | 984 | 51,35 |
| Malva parviflora L. | 960 | 83,78 |
| Arabidopsis thaliana (L.) Heynh. | 925 | 21,62 |
| Fumaria parviflora Lam. | 895 | 64,86 |
| Group 03 : PHI ≤ 500 | ||
| Vicia sativa L. | 200 | 54,05 |
| Papaver hybridum L. | 160 | 27,02 |
| Aethusa cynapium L. | 100 | 32,43 |
Table 3. Partial Harmfulness Index and relative frequency of the most dominant weed species.
These 19 species that are potentially harmful to fodder barley are divided into 3 groups:
Group 01: species with PHI ≥ 1000
This group is made up of 12 species and represents the majority of weeds (63%) that show harmfulness and aggressiveness towards fodder barley. They are considered the most harmful and problematic. Among these weeds, there are 4 perennial species: Lepidium draba, Convolvulus arvensis, Centaurea calcitrapa, and Medicago sativa, two of which are rhizome plants. These weeds are very difficult to control, they have a very high capacity for infestation and propagation in areas larger than their initial area of distribution, and are favored by cultivation practices causing fragmentation of their rhizomes, thus making them more harmful (Hillali in Zidane et al., 2010).
In addition to perennials, annual weeds (Therophytes) are represented by 8 species. Among them, Sinapis arvensis, Sisymbrium irio, and Galium aparine, are reported as the most harmful and problematic of cereals (Zidane et al., 2010; Chafik et al., 2012; Melakhessou et al., 2020; Pala, 2020).
Group 02: 500<PHI<1000
This group is represented by 4 species, or 21%. They are all annuals that propagate by seed, and have adapted well to environments disturbed by repeated cultivation methods each year.
Group 03: PHI ≤ 500
This group includes 3 species, or 15.78%. They are all Therophytes with low average recoveries, but sometimes with medium relative frequencies. This is the case of Vicia sativa. These weeds have less harmfulness compared to the previous groups.
Conclusion
This study made it possible to inventory 36 weed species in irrigated fodder barley crops in Ben Nacer Ben Chohra region. These species belong to 16 families, the most dominant of which are Brassicaceae (22.2%), Asteraceae (19.4%) and Poaceae (13.8%). Dicotyledons predominate (86.2%) and Therophytes (67%) characterize this flora.
The study of infestation degree also showed that 10 species, belonging to general major weeds and general potential weeds groups, form the most infesting weeds in fodder barley crops in this region. In addition, the study of the partial harmfulness index revealed the existence of 19 species which are considered as potentially harmful to fodder barley, 12 of which have a PHI greater than 1000. Combination of the results of infestation degree and those of partial harmfulness index has identified a group of species which constitute a real problem of weeds in Ben Nacer Ben Chohra region. These are Lepidium draba, Sinapis arvensis, Convolvulus arvensis, Centaurea calcitrapa, Sinapis alba, Medicago sativa, Silybum marianum.
This study also revealed that many of the inventoried species can cause poisoning and intoxication in livestock.
Thus, any weed control strategy in this arid region should take into consideration the rational management of these weed species by focusing the fight in particular against the most harmful and the most infesting which could, on the one hand, compete with the fodder barley on space, light, mineral elements and water; and on the other hand, reduce the health quality of the crop. Knowledge of the biology of the most problematic weeds therefore makes it possible to wisely choose the most appropriate weeding processes as well as the appropriate period of intervention in order to optimize the profitability of the applications.
References
Abdelguerfi, A., Laouar, M., M’hammedi Bouzina, M. (2008). Les productions fourragères et pastorales en Algérie: situation et possibilités d’amélioration. Agriculture and Développement, 6:14-25.
Agaie, B.M., Salisu, A., Ebbo, A.A. (2007). A survey of common toxic plants of livestock in Sokoto State, Nigeria. Scientific Research and Essay, 2:40-42.
Agence Nationale de Sécurité Sanitaire (ANES). (2021). Plantes toxiques en cas d’ingestion. Cedex, France, p:39.
Allison, C.D., Turner, J.L., Wenzel, J.C. (2016). Poisonous plants of New Mexico rangelands. The Linebery Policy Center for Natural Resource Management. Circular, 678:175.
Association de Coordination Technique Agricole (ACTA). (1997). Mauvaises herbes des cultures. Ed. Le Carrousel. Paris, France, p:465.
Barralis, G. (1976). Méthodes d'étude des groupements adventices des cultures annuelles, application à la Côte d'Or. 5ème Colloque International sur l'écologie des mauvaises herbes. Dijon, France, pp:59-68.
Belanger, F. (2020). Les plantes toxiques pour les ruminants. Ministère de l’agriculture, des pêcheries et de l’alimentation. Québec, Canada, p:60.
Bond, W., Davies, G., Turner, R. (2007). The biology and non-chemical control of Charlock (Sinapis arvensis L.). HDRA, The Organic Organisation, p:10.
Bonte, J.B. (2010). La rotation des cultures dans les systèmes céréaliers biologiques: peut-on combiner performances économiques, agronomiques et environnementales Première approche d’analyse multicritères. Mémoire d’Ing, Paris, France, p:61.
Bouhache, M., Boulet, C. (1984). Etude floristique des adventices de la tomate dans le Souss. Hommes Terre Eaux, 14:37-49.
Braun-Blanquet, J. (1952). Les groupements végétaux de la France méditerranéenne. Ed Centre National de la Recherche Scientifique. France, p:297.
Chafik, Z., Berrichi, A., Taleb, A. (2012). Etude des mauvaises herbes des céréales dans la plaine de la Moulouya (Maroc). Revue Marocaine de Protection des Plantes, 3:1-12.
Chehma, A. (2006). Catalogue des plantes spontanées du Sahara septentrional algérien. Laboratoire de recherche: protection des écosystèmes en zones arides et semi-arides. Université de Ouergla. Algérie, p:141.
Chicouene, D. (1999). Evaluation du peuplement de mauvaises herbes en végétation dans une parcelle: Aperçu des méthodes utilisables. Phytoma-Défense des Cultures, 522:22-24.
Daoudi, A., Colin, J.P., Baroud, K. (2021). La politique de mise en valeur des terres arides en Algérie: une lecture en termes d’équité. Cah Agriculture, 30:4.
Di Tomaso, J.M., Kyser, G.B. (2013). Weed control in natural areas in the Western United States. Weed Research and Information Center. University of California, p:544.
Fenni, M. (2003). Étude des mauvaises herbes céréales d’hiver des Hautes Plaines Constantinoises. Écologie, dynamique, phénologie et biologie des bromes. UFA Sétif, p:165.
Francis, A., Warwick, S.I. (2007). The biology of canadian weeds. 3. Lepidium draba L., L. chalepense L., L. appelianum Al-Shehbaz (updated). Canadian Journal of Plant Science, 88:379-401.
Graves-Medley, M., Mangold, J. (2018). Biology, ecology and management of whitetop (Lepidium spp.). Montana State University, p:11.
Guerin, M. (2016). Le liseron des champs: biologie, impact, gestion. Center for Landscape and Urban Horticulture, p:11.
Hadbaoui, I., Senoussi, A., Huguenin, J. (2020). Les modalités d’alimentation des troupeaux ovins en steppe algérienne, région de M’Sila: pratiques et tendances. Cah Agriculture, 29:28.
Hall, A.L., Gornish, E., Ruyle, G. (2020). Poisonous plants on rangelands. The University of Arizona Cooperative Extension, p:10.
Hamrit, S. (1995). Situation des fourrages en Algérie. Al-Awamia, 89:97-108.
Hannachi, A., Fenni, M. (2013). Etude floristique et écologique des mauvaises herbes des cultures de la région de Batna (Algérie). Revue Agriculture, 5:24-36.
Issolah, R. (2008). Les fourrages en Algérie: situation et perspectives de développement et d’amélioration. Recherche Agronomique, 22:34-47.
Jacobs, J. (2007). Ecology and management of field bindweed (Convolvulus arvensis L.). NRCS-Montana-Technical Note-Invasive Species-MT, 9:9.
Ka, S.L., Sarr, M., Gueye, M., Mbaye, M.S., Noba, K. (2020). Degré d’infestation et nuisibilité potentielle des mauvaises herbes du sorgho (Sorghum bicolor) en Haute Casamance, Sénégal. Review Marine Science Agronomy Vét, 8:301-306.
Karkour, L., Fenni, M. (2016). Effet des pratiques culturales sur la dynamique des flores adventices des terres cultivées dans la zone semi-aride (Algérie). Premier séminaire international sur: Systèmes de production en zones semi-arides. Diversité Agronomique et Systèmes de Cultures. M’sila. Revue Agriculture, pp:25-61.
Keywanloo, M., Shahroozian, E., Ahmadi-Hamedani, M., Javaheri-Vayeghan, A., Emadi Chashmi, H. (2021). Possible acute poisoning by Sinapis arvensis in sheep: the clinical, laboratory and necropsy findings. Archives of Razi Institute, 76:699-706.
Le Bourgeois, T. (1993). Les mauvaises herbes dans la rotation cotonnière au Nord-Cameroun (Afrique): amplitude d'habitat-degré d'infestation. Thèse de Doctorat, Montpellier II. Montpellier, France, p:250.
Le Chatelier, D., Joya, R., Martinet, Y. (2016). Les légumineuses, alliées d’une agriculture écologiquement intensive. L’exemple de la luzerne. Pollution Atmosphérique, pp:220-222.
Maillet, J. (1981). Evolution de la flore adventice dans le Montpelliérais sous la pression des techniques culturales. Thèse de DDI, USTL. Montpellier, France, p:200.
Main, D.C., Butler, A.R. (2006). Probable Malva parviflora (Small flowered mallow) intoxication in sheep in Western Australia. Australian Veterinary Journal, 84:134-135.
Marnotte, P. (1984). Influence des facteurs agro-écologiques sur le développement des mauvaises herbes en climat tropical humide. 7ème College Int. Ecology Biology et Systems des Mauvaises Herbes. Paris, France, pp:183-189.
Melakhessou, Z., Demnati, F., Boubaker, Z. (2020). Diagnostic de la diversité des plantes adventices dans les agrosystèmes: cas des champs de blé dans les Aurès. Bulletin de la Société Royale des Sciences de Liège, 89:39-54.
Mohammedi, D., Mohammedi, S., Keck, G. (2014). Principales intoxications végétales chez les ruminants en zone méditerranéenne. Revue d’élevage et de Médecine Vétérinaire des Pays Tropicaux, 67:163-171.
Mulligan, G.A., Munro, D.B. (1990). Poisonous plants of Canada. Canadian Government Publishing Centre. Ottawa, Canada, p:96.
Ozenda, P. (1991). Flore et végétation du Sahara. Centre National de la Recherche Scientifique. Paris, France, p:662.
Pala, F. (2020). Observation of weed species, frequency and density in common barley (Hordeum vulgare L.) fields of Diyarbakir, Turkey: A Case Study. Journal of Agricultural Sciences, 26:164-172.
Quezel, P., Santa, S. (1962). Nouvelle flore de l’Algérie et des régions désertiques méridionales, Tome 01. Centre National de la Recherche Scientifique. Paris, France, p:558.
Quezel, P., Santa, S. (1963). Nouvelle flore de l’Algérie et des régions désertiques méridionales, Tome 02. Centre National de la Recherche Scientifique. Paris, France, p:571-1170.
Raunkiaer, C. (1905). Types biologiques pour la géographie botanique. Bulletin Academic Danemark, 5:347-437.
Sosnoskie, L.M., Hanson, B.D., Steckel, L.E. (2020). Field bindweed (Convolvulus arvensis): all tied up. Weed Technology, 34:916-921.
Stubbendieck, J., Carlson, M.P., Dunn, C.D., Anderson, B.E., Redfearn, D. (2018). Nebraska plants toxic to livestock. Including bloat-causing plants rangeland, pasture land, and crop land. Institute of Agriculture and Natural Resources. University of Nebraska-Lincoln, p:196.
Taleb, A., Bouhache, M., Rzozi, S.B. (1997). Etude de la flore adventice de la canne à sucre dans la région du Loukkos. Actes Institute Agronomy Vet, 17:103-108.
Tanji, A., Ait Lhaj, A. (2010). Adventices de l’orge et du blé dans la région de Souss-Massa. Revue Marocaine de Protection des Plantes, 1:11-23.
Traore, H., Maillet, J. (1998). Mauvaises herbes des cultures céréalières au Burkina Faso. Agriculture et Développement, 20:47-59.
Warwick, S.I., Beckie, H.J., Thomas, A.G., Mcdonald, T. (2000). The biology of canadian weeds. 8. Sinapis arvensis L. (updated). Canadian Journal of Plant Science, 80:939-961.
Zidane, L., Salhi, S., Fadli, M., El Antri, M., Taleb, A., Douira, A. (2010). Etude des groupements d’adventices dans le Maroc occidental. Biotechnology Agronomy Social Environment, 14:153-166.
Author Info
M. Hattab*Citation: Hattab, M. (2022). Study of infestation and harmfulness degree of weeds on fodder barley (Hordeum vulgare L.) in an arid region (Laghouat-Algeria). Ukrainian Journal of Ecology. 12:66-73.
Received: 19-Feb-2022, Manuscript No. UJE-22-55026; Accepted: 14-Mar-2022, Pre QC No. P-55026; Editor assigned: 23-Feb-2022, Pre QC No. P-55026; Reviewed: 01-Mar-2022, QC No. Q-55026; Revised: 07-Mar-2022, Manuscript No. R-55026; Published: 21-Mar-2022, DOI: 10.15421/2022_355
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
