Research - (2023) Volume 13, Issue 1
Systematic and ecological study of mosquitoes (Diptera: Culicidae) at lake Fetzara (Annaba, Northeast Algeria)
M. Boulares1, N. Rehimi1, I. Houhamdi2, A. Baaloudj2*, N. Soltani1 and M. Houhamdi2Abstract
The aim of this study is to identify the different species of Culicidae dispersed in Lake Fatazara (Annaba, Northeast of Algeria). The systematic study was conducted in this lake during the 12 months of 2016 when the collection of specimens of Culicidae was being carried out once a month in rural sites. Species were identified using the software of Schaffner et al., (2001) and Culicidae from Mediterranean Africa. The results obtained showed us the presence of six species scattered all around the three surveyed deposits representing five genera were identified: Culex tritaeniorhynchus (49.52%); Culiseta longiareolata (24.87%); Culex pipiens (18.95%); Orthopodomyia pulcripalpis (3.27%); Aedes punctor (3.27%) and Aedesvexans (0.1%).
Keywords
Lake Fezzara, Systematic, Specimens, Diversity, Ecology.
Introduction
Mosquitoes have a cosmopolitan distribution. There are about 3,500 species and subspecies, fewer than 140 subgenera in 42 genera of mosquitoes worldwide. (Walter Reed Biosystematics Unit, 2001; Rueda, 2008). Yet, there are probably more than 1,000 species to be found and described (Rueda, 2008). Insects in many parts of the world, create, by their bites, a considerable nuisance. Moreover, and more seriously, they carry many diseases, especially in the tropics where they cause significant morbidity and mortality. The largest group of biting Diptera is mosquitoes. Mosquitoes play an important role in the transmission of a number of tropical diseases such as malaria, filariasis, and several viruses, dengue fever, Japanese encephalitis, and yellow fever in particular. In temperate countries, they are more of a nuisance than vectors of disease. For almost two decades a very important and diverse number of works has been done on the culicidian fauna of Algeria that is particularly interested in the systematics, biochemistry, morphometry, chemical and biological control against mosquitoes (Bendali et al., 2001; Boudjelida et al., 2005; Tine-Djebbar and Soltani, 2008; Tine-Djebbar, 2009; Messai et al., 2011; Tine-Djebbar et al., 2011). Among the works which are concerned with the systematics of mosquitoes are: Messai et al., (2011); Merabti and Ouakid, (2011); Bouabida et al., (2012), Berchi et al., (2012), Tahraoui, (2012), Aissaoui, (2014), Boudemagh et al., (2013), Oudainia, (2015); Lounaci, (2015); Benmalek et al., (2017); Dahchar et al., (2017); Houmani et al., (2017); Hamaidia and Berchi, (2018); Benhissen et al., (2014, 2018), Serradj et al., (2018), Nabti and Bounechada, (2019), Chahed et al., (2021), Arrousi et al., (2021). The real danger of mosquitoes is the reason that requires us urgent intervention to conduct a systematic and ecological study to determine the harmful species and to start a biological fight with essential oils extracted from aromatic plants for the reduction of mosquito proliferation in our region.
Materials and Methods
Presentation of the study area: Lake Fetzara is located in the northeast of Algeria, 18 km southwest of the city of Annaba. It stretches for 17 km from East to West and 13 km from North to South with an area of about 18600 ha. The Edough massif to the north borders this lake, the hills of Ain Berda to the south, and the dune ridges to the east and west. All around Fetzara Lake, there are several settlements, the commune of Berrahal to the North, the municipalities of El-Eulma and Cheurfa to the South, and the small villages of El Gantra and Oued Zièd to the East (Fig. 1).
Fig 1: lgpirpargheg ehT location of lake Fetzara.
In the course of our study, the pre-imaginary stages were sampled using a 1000 ml plastic ladle and the harvested larvae are deposited in 5-liter water bottles with tags (the date and the site). The Larvae and nymphs were counted and sorted. The nymphs were placed in containers containing water from the survey, then deposited in cages containing the food of the adults (dates) until emergence. While larvae were reared in 150 ml containers of dechlorinated water and fed with 0.04 g of the 75% to 25% yeast cookie mixture (Rehimi and Soltani, 1999). The water is renewed after a few days. The resulting nymphs are transferred into cages until the adults emerge to perform our mass breeding by giving them a blood meal for the development of eggs. The larvae of the 4th stage are put in a 10% NaOH solution for 24 hours until the desired enlightenment takes effect in order for us then to make the identification.
Larvae, with their hose determination characteristics, are often microscopic and must be mounted between slide and coverslip, identification of the genus and species has been made under a binocular microscope, using the last larval stage (L4) and confirmation by the adult according to morphological criteria defined by a Culicidae identification software, that of Scaffner et al., (2001) "The Mosquitos of Europe"
Method of exploitation of results by ecological indices
Our results were expressed through ecological indices of composition, which are characterized by specific richness, relative abundance, and frequency of occurrence on the one hand, and on the other hand through ecological indices of structure, which are the Shannon-Whpvpgi ndpx,iS mesan’siIndpx,iH TTiIndpx,ihndiCgpwiIndpx.
Results and Discussion
The Analysis of the composition of the Culicidian population of the study sites reveals the presence of six Culicidae species belonging to a single subfamily: Culicidae. Where four tribes were found: The tribe of the Aedini is represented by two species: Aedes pinctor and Aedes vexans Culicini tribe comprise a single genus, and that of Culex is represented by two species: Culex tritaeniorhynchus and pipiens, Orthopodomyini tribe with a single species Orthopodomyia pulchripalpis, and finally the Culisetini tribe represented by a single species, Culiseta longiareolata. The specific wealth values for the three stations studied are shown in Table 1. According to the values of specific richness, station 3 takes first place, with a maximum richness of Culicidian species of three, followed by other stations 1 and 2 with a richness of only two species. The relative abundance values for the different species are reported in Table 2. The results show relative abundance values that vary from species to species by the population at the three study stations. Cx. Tritaeniorhynchus was the dominant species with a rate of 49.52%, then comes Cs. Longiareolata with 24.87%, followed by Cx. Pipienswith 18.95%. There is an equal percentage between Or. Pulcripalpisand Ae. Punctorwith 3.27% and lesser importance for Ae. Vexans with 0.1%.
| Site Species |
Fetzara lake | ||
|---|---|---|---|
| Station 1 | Station 2 | Station3 | |
| RS | 02 | 02 | 03 |
| Cx. tritaeniorhynchus | 49.52 | - | - |
| Or. pulcripalpis | 3.27 | - | - |
| Cx. pipiens | - | 18.95 | - |
| Cs. longiareolata | - | 12.32 | 12.57 |
| Ae. punctor | - | - | 3.27 |
| Ae. vexans | - | - | 0.1 |
Table 1. Relative abundance of species collected at the three stations studied. Abondance relative des espèces récoltées dans les trois stations étudiées.
| Site Parameters and species |
Fetzara lake | ||
|---|---|---|---|
| Station 1 | Station2 | Station3 | |
| Cx. tritaeniorhynchus | 985 | - | - |
| Or. pulcripalpis | 65 | - | - |
| Cx. pipiens | - | 377 | - |
| Cs. longiareolata | - | 245 | 250 |
| Ae. punctor | - | - | 65 |
| Ae. vexans | - | - | 02 |
| Effectif/Station | 1050 | 622 | 317 |
| H’/station | 0.48 | 0.52 | 0.40 |
| S/station | 02 | 02 | 03 |
| H’ max | 01 | 01 | 1.58 |
| E/station | 0.48 | 0.52 | 0.28 |
Table 2. Shannon-weaver diversity and equitability index of culicidian species harvested at the 3 stations studied.
The Shannon-Weaver index is the first calculated index. The calculation steps are listed in Table 2. The value of the Shannon-Weaver index is 0.48 bits in the first station and 0.52-0.40 in the second and third stations. It is found that the Shannon-Weaver values are lower than the maximum equal diversity (1-1-1.58 bits) which shows that the Culicidian population at the three stations studied is not diversified. The low number of mosquito species inventoried in general in our work compared to other works is due to the faunal composition that would influence the development of mosquito larvae in this roost (Saotoing et al., 2014). In addition, the high level of salinity has a negative influence on larval survival . The salinity is directly proportional to the conductivity and according to the work of ZAHI et al., 2013; which shows a significant variability of values between 350 and 3500 µS/cm with a majority between 350 and 1000 µS/cm. Mosquitoes are found only in less salty oceanic areas of the lake.
There has been a clear disappearance of Cx. Tritaeniorhynchus in all the work of the last twenty years. Despite that, this species is most abundant in our work and has been mentioned in the work of Brunhes, (2000). This absence is justified by the fact that this species is less diverse in Algeria.
The revelation of Cs. Longiareolata in the two sites and his presence in almost all the works (Merabti and Ouakid.,2011; Messai et al., 2011; Bouabida et al., 2012; Berchi et al., 2012; Aissaoui, 2014; Boudemagh et al., 2013; Oudainia, 2015; Lounaci, 2015; Dahchar et al., 2017; Hamaidia and Berchi, 2018; Benhissen et al., 2014, 2018; NABTI and Bounechada, 2019; Chahed et al., 2021) put Cs. Longiareolata, the second most widespread species after Cx. pipiens. All the systematic work in Algeria shows that Cx. pipiensis the most widely distributed species in Mediterranean Africa (Dahchar et al., 2017), by the work of (Merabti and Ouakid, 2011; Messai et al., 2011; Bouabida et al., 2012; Berchi et al., 2012; Tahraoui, 2012; Aissaoui, 2014; Boudemagh et al., 2013; Oudainia, 2015; Lounaci, 2015; Benmalek et al., 2017; Dahchar et al., 2017; Houmani et al., 2017; Hamaidia and Berchi, 2018; Benhissen et al., 2014, 2018; Serradj et al., 2018; NABI and Bounechada, 2019; Chahed et al., 2021). The species of Orthopodomyia pulcripalpisis found in some of the work of (Boudemagh et al., 2013; Oudainia, 2015; Dahchar et al., 2017; Hamaidia and Berchi., 2018) which justifies its low percentage in our work. Ae. Vexans revealed in low percentage and its distribution known by the works (Merabti and Ouakid, 2011; Tahraoui, 2012; Benhissen et al., 2014; Aissaoui, 2014, Houmani et al., 2017). That the work of Hamaidia in Souk Ahras in the last ten years shows the existence of Ae. punctor (Hamaidia and Berchi, 2018). The lodgings of Lake Fetzara are marshes and are almost identical to each other and each one of them has its own faunistic particularity. The ecological study requires a thorough knowledge of ecological factors, and according to (Dajoz, 1971), the climatic factor presented by temperature and precipitation is the important element on which the distribution of Culicidian fauna depends. The results showed a disruption in species revelation every 12 months due to the dry year. Our results, which showed a small number of species (6), do not have an affinity with altitude, which plays a primordial role, and according to (Hassain, 2002), the specific richness of Culicidae in Mediterranean Africa decreases with altitude. This author is to harvest 48 species between 0 and 100m altitude and 20 species between 1000 and 1500 m. The results of (Hadj, et al., 2013) show that the genus Culex persists during all seasons. Culiseta longiareolata and Culex pipiens develop in some breeding sites during the winter. This rare fact could be explained by global warming in North Africa resulting in milder winters, especially during the last decade (Walker and Lynch, 2007). Thus, the warming of certain deposits constitutes a handicap to larval development; this is what is observed in the deposit every three lodgings during the months of January, February and December. In the three breeding sites, the development cycle of all our species is interrupted in the first months of the year 2016, which corresponds to the winter season. This stop in the development of open pits has been observed by several authors (Rioux et al., 1965).
All these prosecutions show that the 3 stations constitute true larval deposits for the Culicidian fauna. (El-Ouali Lalami et al., 2010) indicates that the nature of the larval deposit favors one or the other species depending on whether the deposit is stagnant or currently devoid or rich in vegetation, or polluted or not.
The third site houses a more diversified Culicidian fauna; as a result of the plant diversity of its deposit 03 species have been inventoried. It has a specific diversity, the highest, or the conditions favorable to the installation of the Culicidian fauna. The climatic factor represents the important element on which the distribution of Culicidian fauna depends by temperature and precipitation. These results are consistent with those reported by Tine-Djebbar, (2009).
As regards equitability, the values tending to 1, in the case of the second deposit (0.52), imply that the present species are in equilibrium with each other. The study of equitability suggests that the higher the population is, the more balanced it can be. On the other hand, in deposits that are considered to be small water collections, the equitability values are all less than 0.5 implying the presence of an imbalance between the numbers of species present, this is the case of the G1 and G3 deposits presented by the index 0.48 and 0.28, successively. Their species are out of balance.
Multivariate Statistical Analysis by the AFC (Correspondence Factorial Analysis) performed by ADE software version 4 (Chessel and Doledec, 1992) in its 1 x 2 factorial plane, which gathers 55% and 34%, or 89% of the total inertia (Fig. 2) exposes us the following data:
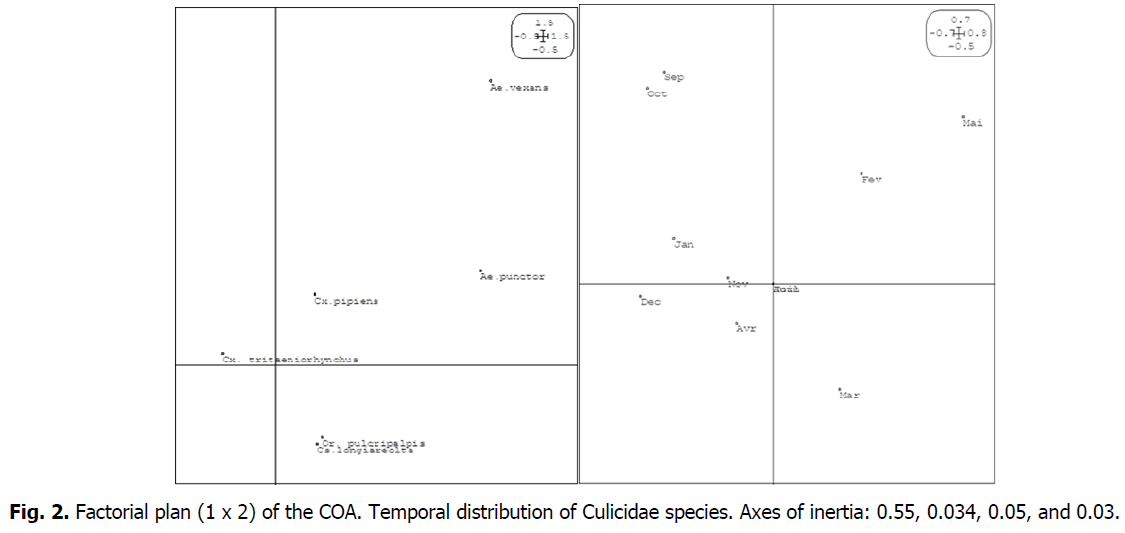
Fig 2: Factorial plan (1 x 2) of the COA. Temporal distribution of Culicidae species. Axes of inertia: 0.55, 0.034, 0.05, and 0.03.
The months of June, July, and August (summer months) are concentrated in the center of the graph showing zero abundance. Factor 1 (Axis of ordinates) contrasts the months of February, March, and April where we observe balanced representativeness in cash, especially Cx. tritaeniorhynchus, Or. pulcripalpis, Ae. punctor, Ae. vexans, Cs. longiareolta, Cx. pipiens. The remaining six months, January, April, August, September, October, November, and December.
Conclusion
The work undertaken in this study represents a first approach to the systematics and morphology of the Culicidian fauna; it is indispensable for the understanding of these taxa. Taxonomic studies at the Lake Fetzara region of Annaba have allowed us to determine 06 species of Culicidae, belonging to 4 genera, Culex, Culiseta, Aedes, and Orthopodomyia, it consists of three sub-families that of Culicini, Aedini Culisetini and Orthopodomyini: Culex pipiens, Culex tritaeniorhynchus, Aedes vexans, Aedes punctor, Culiseta longiareolata, and Orthopodomyia puplcripalpis. The identification of the species inventoried took place in the larval state (stage L4) of the mosquitoes, taking into account all the criteria indicated in the dichotomous key used. Among the inventoried species there is, they have a medical interest (culexpipiens and Culex tritaeniorhynchus) and veterinary interest such as Culiseta longiareolata. The ecological study of the ecological indices showed us the total abundance of Culex tritaeniorhynchus and the rarity of Aedes vexans.
Acknowledgments
Our sincere thanks to the MESRS and the DGRSDT for their help and support and to the entire team of the LBEE laboratory (University 8 May 1945 Guelma) who graciously provided us with the equipment and reagents to carry out our experiments.
References
Linda, A. (2015). Ecophysiological and systematic study of Culicidae in the Tébessa region and biological control (Doctoral dissertation, Badji Mokhtar University).
Arroussi, D.E.R., Bouaziz, A., Boudjelida, H. (2021). Mosquito survey reveals the first record of Aedes (Diptera: Culicidae) species in urban area, Annaba district, Northeastern Algeria. Polish Journal of Entomology, 90:14-26.
Bendali, F., Djebbar, F., Soltani, N. (2001). Comparative efficacy of a few fish species against various stages of Culex pipiens L. under laboratory conditions. Parasitica, 57:255-265.
Wafa, B.S.H., Fatiha, M., Hadjer, M., Laid, O.M., Abdelmadjid, B. (2014). Inventory of Culicidae in Arid Zones: Case of the Ouled-Djellal Oasis (Biskra; Algeria). Revue ElWahat pour les Recherches et les Etudes. 7:86-91.
Benhissen, S., Habbachi, W., Rebbas, K., Masna, F. (2018). Études entomologique et typologique des gîtes larvaires des moustiques (Diptera: Culicidae) dans la région de Bousaâda (Algérie) Entomological and typological studies of larval breeding sites of mosquitoes (Diptera: Culicidae) in Bousaâda area (Algeria). Bulletin de La Société Royale Des Sciences de Liège.
Benmalek, L., Bendali-Saoudi, F., Soltani, N. (2018). Inventory and distribution of mosquitoes (Diptera; Culicidae) of the Burgas lakes (Northeast Algeria). Journal of Entomology and Zoology Studies, 6:838-843.
Berchi, S., Aouati, A., Louadi, K. (2012). Typology of breeding sites favorable to the larval development of Culex pipiens L. 1758 (Diptera-Culicidae), a source of nuisance in Constantine (Algeria). Ecologia Mediterranea , 38:5-16.
Bouabida, H., Djebbar, F., Soltani, N. (2012). Etude systématique et écologique des Moustiques (Diptera: Culicidae) dans la région de Tébessa (Algérie). Entomologie Faunistique-Faunistic Entomology.
Boudemagh, N., Bendali-Saoudi, F., Soltani, N. (2013). Inventory of Culicidae (Diptera: Nematocera) in the region of Collo (North-East Algeria). Annals of Biological Research, 4:94-99.
Boudjelida, H., Bouaziz, A., Soin, T., Smagghe, G., Soltani, N. (2005). Effects of ecdysone agonist halofenozide against Culex pipiens. Pesticide Biochemistry and Physiology, 83:115-123.
Brunhes, J., Hassaïne, K., Rhaiem, A., Hervy, J.P. (2000). Les Culicidés de l'Afrique méditerranéenne: espèces présentes et répartition (Diptera, Nematocera). Bulletin de la Société Entomologique de France, 105:195-204.
Chahed, S., Brahmi, K., Djouaher, T. (2021). Study on the Culicidian fauna (Diptera: Culicidae) of the Tizi-Ouzou region (North of Algeria): Biodiversity, abundance and distribution. Faunistic Entomology-Faunistic Entomology.
Chessel, D., Dolédec, S., Thioulouse, J., Olivier, J.M. (1992). ADE software. Multivariate Analysis and Graphical Display for Environmental Data (version 4). Université de Lyon.
Dahchar, Z., Oudainia, W., Bendali-Saoudi, F., Soltani, N. (2017). Inventory of Culicidae of the wetland (of the West region of Annaba). Journal of Entomology and Zoology Studies, 5:430-436.
DAJOZ, R. (1971). Précis d’écologie. Ed. Dunod, Paris, p:434.
Lalami, A.E.O., El Hilali, O., Benlamlih, M., Merzouki, M., Raiss, N., Koraichi, S.I., Himmi, O. (2010). Etude entomologique, physicochimique et bactériologique des gîtes larvaires de localités à risque potentiel pour le paludisme dans la ville de Fès. Bulletin de l’Institut Scientifique, Rabat, 32:119-127.
Hadji, M., Belghyti, D., El Assal, M., Elomari, F., Rahmoun, H. (2013). Etude entomologique, physico-chimique des gîtes larvaires de Anopheles, Culex. Science Lib, 5:13020626.
Hamaidia, H., Berchi, S. (2018). Systematic and ecological study of mosquitoes (Diptera: Culicidae) in the region of Souk-Ahras (Algeria). Faunistic Entomology-Faunistic Entomology.
Hassain, K. (2002). Biogéographie et biotypologie des Culicidae (Diptera-Nematocera) de l’Afrique méditerranéenne. Bioécologie des espèces les plus vulnérantes (Aedes caspius, Aedes detritus, Aedes mariae et Culex pipiens) de la région occidentale d’Algérie.
Houmani, M., Bendali-Saoudi, F., Soltani, N. (2017). Inventory of Culicidae in the region of El Taref (North-east Algeria). Journal of Entomology and Zoology Studies, 5:263-267.
Merabeti, B., Ouakid, M.L. (2011). Contribution to the study of mosquitoes (Diptera: Culicidae) in the oases of the Biskra region (north-eastern Algeria). Proceedings of the International Seminar on Fauna Biodiversity in Arid and Semi-arid Zones. Ouargla, 4:185-189.
Messai, N., Berchi, S., Boulknafd, F., Louadi, K. (2010). Inventaire systématique et diversité biologique de Culicidae (Diptera: Nematocera) dans la région de Mila (Algérie). Entomologie Faunistique-Faunistic Entomology.
Nabti, I., Bounechada, M. (2020). Mosquito biodiversity in Setif region (Algerian high plains), density and species distribution across two climate zones. Entomologie Faunistique-Faunistic Entomology.
Wafa, O. (2014). Etude bioécologique et systématique des culicidae de la région d’oum el bouaghi effet de la température sur l’agressivité et la biologie de culex pipiens.
Rehimi, N., Soltani, N. (1999). Laboratory evaluation of Alsystin, a chitin synthesis inhibitor, against Culex pipiens pipiens L.(Dip., Culicidae): effects on development and cuticle secretion. Journal of Applied Entomology, 123:437-441.
Rioux, J.A., Croset, H., Gras, G., Juminer, B., Tesson, G. (1965). Les problemes théoriques et pratiques posés par la lutte contre Culex pipiens L. dans le sud de la France. Architecture Institute of Pasteur Tunis, 42:473-500.
Rueda, L.M. (2007). Global diversity of mosquitoes (Insecta: Diptera: Culicidae) in freshwater. In Freshwater Animal Diversity Assessment, Springer, Dordrecht, pp:477-487.
Saotoing, P., Njan-Nloga, A.M., Tchuenguem-Fohouo, F.N., Yaya, O., Messi, J. (2014). Bio-écologie des larves de Culicidae (Diptera) dans la ville de Maroua, Extrême-Nord du Cameroun. International Journal of Innovation and Applied Studies, 39:438-448.
Schaffner, F., Angel, G., Geoffroy, B., Hervy, J.P., Rhaiem, A., Brunhes, J. (2001). Les moustiques d’Europe: logiciel d’identification et d’enseignement. The mosquitoes of Europe: an identification and training programme. IRD Editions and EID Méditerranée: Montpellier, France.
Serradj, N., Bendali-Saoudi, F., Soltani, N. (2018). Inventory of the invertebrate fauna at the level of the lake of Birds (North-east-Algeria). Journal of Entomology and Zoology Studies, 6:98-106.
Shannon, C.E. and Weaver, W. (1963). The mathematical theory of communication. University of Illinois Press.
Tahraoui, C. (2012). Abondance saisonnière des Culicidae dans l’écosystème humide du parc national d’El-Kala: Identification et lutte.
Tine-Djebar, F., Soltani, N. (2008). Activité biologique d'un agoniste non stéroïdien de l’hormone de mue sur Culiseta longiareolata: analyses morphométrique, biochimique et énergétique. Synthèse: Revue des Sciences et de la Technologie, 18:23-34.
Tine-Djebbar, F., Bouabida, H., Soltani, N. (2011). Caractérisation morphométrique et biochimique de certaines espèces de moustiques inventoriées dans la région de Tebessa. Bulletin Social and Zoology France, 136:177-185.
Walker, K., Lynch, M. (2007). Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Medical and Veterinary Entomology, 21:2-21.
Unit, W.R.B. (2001). Systematic Catalog of Culicidae. Smithsonian Institution, Washington, DC, USA.
Zahi, F., Djamai, R., Chaab, S., Djabri, L., Drouiche, A., Medjani, F. (2013). Dynamics of the water table and physico-chemical qualities of the underground waters of Lake Fetzara (north-eastern Algeria). Synthesis: Science and Technology Review, 26:86-95.
Author Info
M. Boulares1, N. Rehimi1, I. Houhamdi2, A. Baaloudj2*, N. Soltani1 and M. Houhamdi22Biology, Water and Environment Laboratory (LBEE), SNV-STU Faculty, University, BP. 401 24000 Guelma, Algeria
Citation: Boulares, M., Rehimi, N., Houhamdi, I., Baaloudj, A., Soltani, N., Houhamdi, M. (2023). Systematic and ecological study of mosquitoes (Diptera: Culicidae) at lake Fetzara (Annaba, Northeast Algeria). Ukrainian Journal of Ecology. 13:1-7.
Received: 03-Dec-2022, Manuscript No. UJE-22-82223; , Pre QC No. P-82223; Editor assigned: 06-Dec-2022, Pre QC No. P-82223; Reviewed: 19-Dec-2022, QC No. Q-82223; Revised: 24-Dec-2022, Manuscript No. R-82223; Published: 04-Jan-2023, DOI: 10.15421/2023_418
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
