Research - (2021) Volume 0, Issue 0
Bioinformatics model of histoenzymatic parameters of the skin glands of Norway (Rattus norvegicus) and black (Rattus rattus) rats
A.B. Kiladze* and N.K. DzhemukhadzeAbstract
Using the Python programming language, the histoenzymatic activity of the skin glands of male and female Norway and black rats was compared, which should be considered in the marking and social behavior of these rodents. Four arrays of digital analogs (24 parameters in each array) containing data on acid phosphatase, alkaline phosphatase, adenosine triphosphatase, and peroxidase were generated for comparison, and whose histoenzymatic activity was studied for skin glands of the nape, the mouth corners, upper eyelids, anal area, and soles of the paws (eccrine and sebaceous glands). The following results have been determined for each variant of the comparison between species and sexes: (1) for the system "male Norway rats-male black rats", it was shown that in four cases (16.7%), the histoenzymatic activity of the skin glands prevailed in male black rats, the dominance of male Norway rats amounted to eleven cases (45.8%), and the equality of the histoenzymatic activity of the skin glands between the two species was revealed in nine cases (37.5%); (2) for the system "male Norway rats-female black rats", it was shown that in five cases (20.8%), the histoenzymatic activity of the skin glands prevailed in female black rats, the dominance of male Norway rats amounted to twelve cases (50.0%), and the equality of the histoenzymatic activity of the skin glands between the two species was revealed in seven cases (29.2%); (3) for the system "female Norway rats-male black rats", it was shown that in five cases (20.8%), the histoenzymatic activity of the skin glands prevailed in male black rats, the dominance of female Norway rats amounted to ten cases (41.7%), and the equality of the histoenzymatic activity of the skin glands between the two species was revealed in nine cases (37.5%); (4) for the system "female Norway rats-female black rats", it was shown that in three cases (12.5%), the histoenzymatic activity of the skin glands prevailed in female black rats, the dominance of female Norway rats amounted to eight cases (33.3%), and the equality of the histoenzymatic activity of the skin glands between the two species was revealed in thirteen cases (54.2%). In all four interaction systems, the dominance of Norway rats over black rats in skin glands histochemical activity was established.
Keywords
Norway (brown) rat, black rat, interspecific relationships, skin, glands, histochemistry, computational biology.
Introduction
The study of interspecific interactions of Norway and black rats is of significant interest for biological science given both the isolation and co-existence of individuals, which may have not only the character of mutual vital activity but also competitive relations (Figure 1) for food resources, territorial niche and other aspects having an ecological character (Kalinin, 1995; King et al., 2011; Feng & Himsworth, 2014). Chemocommunication interaction is carried out at the level of chemical signal sources, including various skin glands, both specific and nonspecific (Quay, 1986; Bell, 1986; Hashimoto et al., 1986). Interspecific interaction can be carried out according to various ethological scenarios and mechanisms, while the skin glands are responsible for marking behavior, which is relatively stable and developed in rats (Dzhemukhadze, 2007).

Figure 1: Norway rat (Rattus norvegicus) attacked black rat (Rattus rattus). Taxidermy mounts. The Zoological Museum of the Zoological Institute of the Russian Academy of Sciences. Saint Petersburg. Photo by A.B. Kiladze.
In this regard, modeling the chemocommunication interaction of Norway and black rats is an urgent task for zoology and ethology (Schweinfurth, 2020). Given that the enzyme activity levels are chosen as the physiological parameters that are put into the model of interaction between individuals, this work may also be of interest for histoenzymology (Lojda et al., 1979; Lyon & Barer, 1991; Van Noorden & Fredericks, 1992).
At present, it is important to use accurate methods that allow for analyzing the physiological parameters related to the quantification of the chemocommunication interaction between different-sex individuals belonging to two rat species (Kiladze & Dzhemukhadze, 2020). To achieve this goal, several bioinformatics methods can be used, which are diverse and have a wide range of possibilities for interpreting biological data (Pereira et al., 2021).
Bioinformatics, being a part of computational biology (Moseley, 2020), will allow us to find some quantitative parameters that characterize the interspecific interaction of Norway and black rats (Foster, 2010). It is necessary to treat these results with a certain degree of assumption since the identified relationships can only be correlated and interpreted to the fundamental functional and biological processes of living systems and behavioral acts.
The enzyme systems ensuring active metabolism of the cells of the glandular structures of the skin have a certain significance in forming and releasing the secretion as a natural metabolite (Saga & Morimoto, 1995; Saga, 2001, 2002; Cui & Schlessinger, 2015), which emphasizes the need to study the degree of histoenzymatic activity, which differs not only in the opposite-sex individuals of the same species but also has signs of interspecific variability (Dzhemukhadze, 2007).
The work aims to demonstrate interspecific variability in the histochemical activity of the skin glands of Norway and black rats.
Materials and Methods
We studied 16 mature individuals (10 males and 6 females) of the Norway rat Rattus norvegicus Berkenhout, 1769, as well as 15 mature individuals of the black rat Rattus rattus Linnaeus, 1758 (9 males and 6 females), captured in Moscow and the Moscow region. The individuals were provided by the vivarium of the A. N. Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences. All institutional and national ethical guidelines for the care and use of laboratory animals were followed. The sebaceous glands were examined from the nape, the mouth corners, the upper eyelids, the anal area, and the soles of the paws. In addition, the eccrine glands of the soles of the paws were studied. Skin samples were fixed in 10% neutral formalin in the cold for up to 24 hours. The thickness of the frozen "floating" sections was 15 µm. Qualitative histochemical reactions to acid phosphatase (ACP), alkaline phosphatase (ALP), adenosine triphosphatase (ATPase), and peroxidase were performed according to the methods given in Pears (1960) and Burstone (1962) manuals. Histoenzymatic activity was expressed in standard (sign) and qualimetric (Azgaldov & Kostin, 2011) forms in the following gradations (digital analogs in points): 0 — "-" — traces or lack of activity; 1 — "-(+)" — indistinct enzyme activity; 2 — "+(-)" — low enzyme content; 3 — "+" — moderate enzyme content; 4 — "++" — the average enzyme content; 5 — "+++" — high enzyme content (Dzhemukhadze & Kiladze, 2008; Kiladze & Dzhemukhadze, 2019).
Digital analogs of histoenzymatic activity were formed into data arrays using the NumPy library in the Python program. The code with a specific sequence of actions on data arrays is given in the "Results" section.
Results
The study design is based on the interaction matrix between males and females belonging to different rat species, as shown in Figure 2, that is, four variants of interaction will be considered: (1) Male Norway rats and male black rats; (2) Male Norway rats and female black rats; (3) Female Norway rats and male black rats; (4) Female Norway rats and female black rats.

Figure 2: Matrix of interspecific interactions of Norway (R. norvegicus) and black (R. rattus) rats.
The order of presentation of the results is as follows: (1) Comparative histochemical analysis of the skin glands enzyme activity (Figure 3) presented in the form of sign and digital analogs; (2) Converting digital analogs to a data array using the Python computer program; (3) Calculation of the difference between the available data sets; (4) Determination of the coincidence and variability of histochemical parameters in interspecific interactions of Norway and black rats; (5) Discussion of the results obtained from a biological point of view.
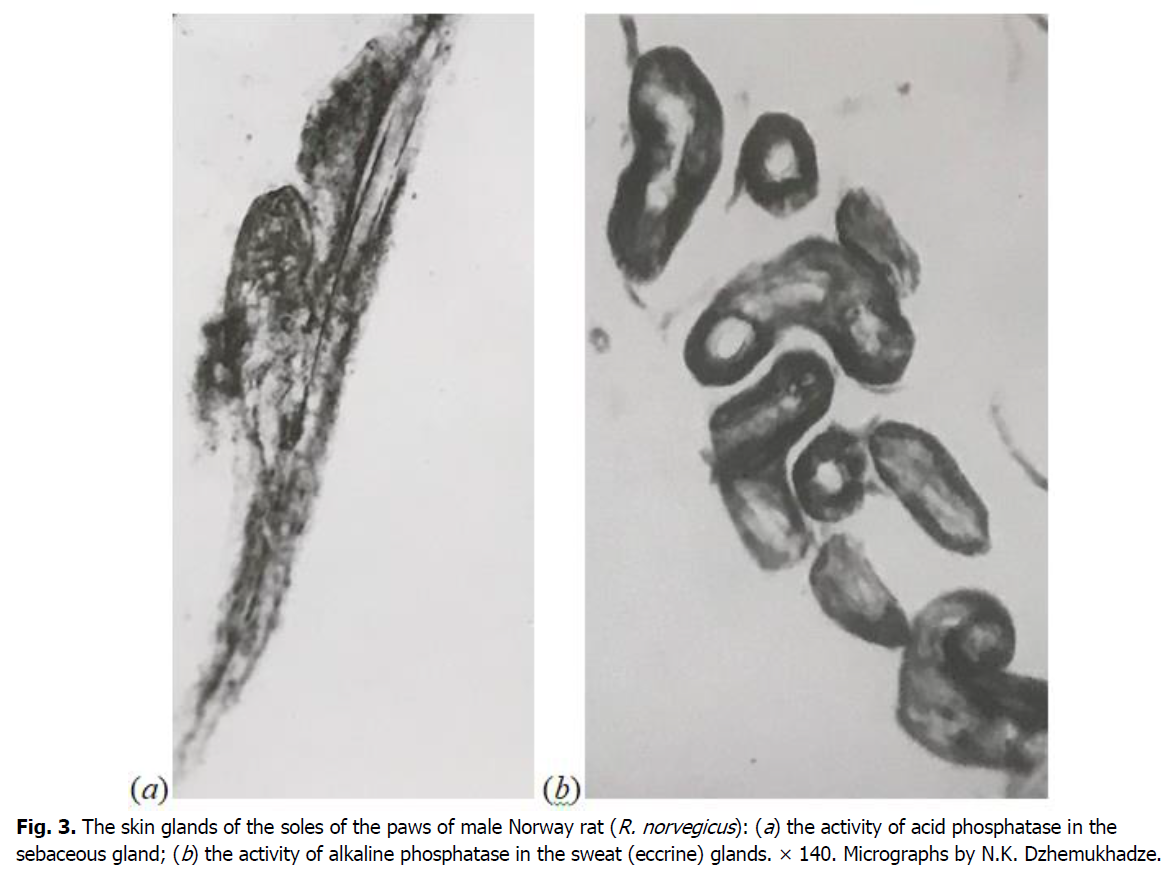
Figure 3: The skin glands of the soles of the paws of male Norway rat (R. norvegicus): (a) the activity of acid phosphatase in the sebaceous gland; (b) the activity of alkaline phosphatase in the sweat (eccrine) glands. × 140. Micrographs by N.K. Dzhemukhadze.
Male Norway rats and male black rats
A comparison of the histoenzymatic activity of the skin glands of male Norway rats and male black rats is shown in Table 1.
| ACP, signs/points |
ALP, signs/points |
ATPase, signs/points |
Peroxidase, signs/points |
||||
|---|---|---|---|---|---|---|---|
| +/3 | ++/4 | ++/4 | +/3 | +/3 | -/0 | +(-)/2 | -/0 |
| ++/4 | ++/4 | +/3 | +/3 | +/3 | +/3 | +(-)/2 | -/0 |
| +/3 | +++/5 | +++/5 | +/3 | ++/4 | ++/4 | -/0 | -/0 |
| +(-)/2 | -(+)/1 | +++/5 | ++/4 | ++/4 | -(+)/1 | +(-)/2 | -/0 |
| +/3 | ++/4 | +/3 | +/3 | +(-)/2 | +(-)/2 | -(+)/1 | -(+)/1 |
Table 1. Comparative analysis of the histoenzymatic activity of the skin glands of male Norway rats (R. norvegicus) and male black rats (R. rattus).
The activity of ACP in the skin glands of the nape, the mouth corners, and in the sebaceous glands of the soles of the paws is characterized by a moderate level in male Norway rats and an average level in male black rats. The average activity of ACP for both rat species is characteristic of the upper eyelid glands. The activity of ACP in the anal glands shows a moderate level in male
Norway rats and a high level in male black rats. ACP in the eccrine glands of the soles of the paws is characterized by a low level of activity in male Norway rats and indistinct activity in male black rats.
The activity of ALP in the skin glands of the nape has an average level in male Norway rats and an indistinct character of activity in male black rats. ALP activity for the glands of the mouth corners differs, with the average level for male Norway rats and the moderate level for male black rats. Moderate activity of ALP is characteristic of the glands of the upper eyelids and the sebaceous glands of the soles of the paws in both species of rats. ALP activity in the anal glands is characterized by a high level in male Norway rats and a moderate level in male black rats. ALP activity in the eccrine glands of the soles of the paws has a high level in male Norway rats and an average level in male black rats.
ATPase activity does not reach high values in any of the topographical areas of the skin in both male Norway rats and male black rats. ATPase activity in the skin glands of the nape is low in male Norway rats and indistinct in male black rats. The glands of the mouth corners differ in that they show moderate ATPase activity in male Norway rats, while in male black rats, ATPase is not detected; that is, there is no activity. In both species of rats, the glands of the upper eyelids are characterized by a moderate level of ATPase activity, the anal glands-an average level and the sebaceous glands of the soles of the paws-a a low level. The eccrine glands of the soles of the paws are characterized by an average level of ATPase activity in male Norway rats and indistinct activity in male black rats.
The activity of peroxidase is characterized by the lowest level among other studied enzymes. Thus, in male black rats, almost all the glands from the studied topographic areas do not detect peroxidase (lack of activity). The indistinct activity of peroxidase is manifested only in the sebaceous glands of the soles of the paws. The activity of peroxidase in male Norway rats is not shown (lack of activity) in the skin glands of the nape and in the anal glands, has indistinct activity in the sebaceous glands of the soles of the paws, and also has a low level of activity in the glands of the mouth corners, upper eyelids and in the eccrine glands of the soles of the paws.
Based on the available digital analogs of the histoenzymatic activity of the skin glands of male Norway rats and male black rats, it is advisable to create two data arrays then find the difference between them. The difference found reflects the interspecific parity (el=0), the dominance of male Norway rats (el>0), or the dominance of male black rats (el<0). To do this, the above three outcomes were provided in the "for" loop. The source code has the following sequence of actions:
.. code:: ipython3 import numpy as np
.. code:: ipython3
male_Norway_rats=np.array(
[
[3, 4, 2, 0], [3, 4, 3, 2], [4, 3, 3, 2], [3, 5, 4, 0], [2, 5, 4, 2], [3, 3, 2, 1] ])
male_Norway_rats
.. parsed-literal:: array([[3, 4, 2, 0], [3, 4, 3, 2], [4, 3, 3, 2], [3, 5, 4, 0], [2, 5, 4, 2], [3, 3, 2, 1]])
.. code:: ipython3
male_Black_rats=np.array(
[
[4, 1, 1, 0], [4, 3, 0, 0], [4, 3, 3, 0], [5, 3, 4, 0], [1, 4, 1, 0], [4, 3, 2, 1] ]) male_Black_rats
.. parsed-literal:: array([[4, 1, 1, 0], [4, 3, 0, 0], [4, 3, 3, 0],
[5, 3, 4, 0], [1, 4, 1, 0], [4, 3, 2, 1]])
.. code:: ipython3
male_Norway_rats - male_Black_rats
.. parsed-literal::
array([[-1, 3, 1, 0], [-1, 1, 3, 2], [ 0, 0, 0, 2], [-2, 2, 0, 0], [ 1, 1, 3, 2], [-1, 0, 0, 0]])
.. code:: ipython3
for el in (male_Norway_rats - male_Black_rats).flat: if el == 0:
print("Interspecific parity (el=0)") elif el > 0:
print("Dominance of male Norway rats (el > 0)") elif el < 0:
print("Dominance of male Black rats (el < 0)")
.. parsed-literal::
Dominance of male Black rats (el < 0) Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Interspecific parity (el=0)
Dominance of male Black rats (el < 0) Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Interspecific parity (el=0)
Interspecific parity (el=0) Interspecific parity (el=0)
Dominance of male Norway rats (el > 0) Dominance of male Black rats (el < 0) Dominance of male Norway rats (el > 0) Interspecific parity (el=0)
Interspecific parity (el=0)
Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Dominance of male Black rats (el < 0)
Interspecific parity (el=0) Interspecific parity (el=0) Interspecific parity (el=0)
The resulting data can be presented in the form of three outcomes with a certain frequency of occurrence. For this purpose, it is advisable to present using a polygon (Figure 4).
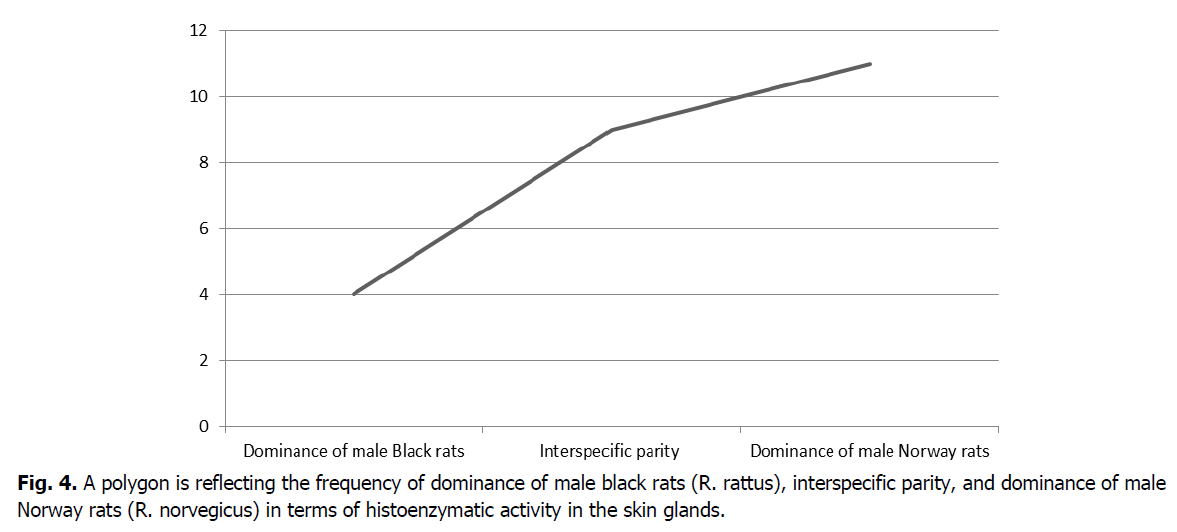
Figure 4: A polygon is reflecting the frequency of dominance of male black rats (R. rattus), interspecific parity, and dominance of male Norway rats (R. norvegicus) in terms of histoenzymatic activity in the skin glands.
Based on the above data, it can be seen that in four cases (16.7%), the histoenzymatic activity of the skin glands prevails in male black rats. In male Norway rats, the dominance of the histochemical parameters of the skin glands amounted to eleven cases (45.8%). The parity ratio indicating the equality of the values of the histoenzymatic activity of the skin glands in male Norway and black rats amounted to nine cases (37.5%). Thus, the data obtained indicate the prevalence of histoenzymatic activity of the skin glands of male Norway rats over male black rats.
Male Norway rats and female black rats
A comparison of the histoenzymatic activity of the skin glands of male Norway rats and female black rats is shown in Table 2.
| Skin glands | ACP, signs/points |
ALP, signs/points |
ATPase, signs/points |
Peroxidase, signs/points |
||||
|---|---|---|---|---|---|---|---|---|
| Female Norway rats | Female black rats | Female Norway rats | Female black rats | Female Norway rats | Female black rats | Female Norway rats | Female black rats | |
| Nape | +/3 | +/3 | ++/4 | +/3 | +(-)/2 | -(+)/1 | -/0 | -/0 |
| Mouth corners | +/3 | +/3 | ++/4 | ++/4 | +/3 | -/0 | +(-)/2 | -(+)/1 |
| Upper eyelids | ++/4 | +/3 | +/3 | +/3 | +/3 | +/3 | +(-)/2 | -/0 |
| Anal area | +/3 | ++/4 | +++/5 | ++/4 | ++/4 | +/3 | -/0 | -(+)/1 |
| Eccrine glands of the soles of the paws | +(-)/2 | ++/4 | +++/5 | +/3 | + +/4 | +(-)/2 | +(-)/2 | -(+)/1 |
| Sebaceous glands of the soles of the paws | +/3 | +/3 | +/3 | ++/4 | +(-)/2 | +/3 | -(+)/1 | -/0 |
Table 2. Comparative analysis of the histoenzymatic activity of the skin glands of male Norway rats (R. norvegicus) and female black rats (R. rattus).
The activity of ACP does not reach high values in any of the topographical areas of the skin in both male Norway rats and female black rats. The activity of ACP in the skin glands of the nape, the mouth corners, and the sebaceous glands of the soles of the paws is moderate in both male Norway rats and female black rats. The average level of ACP activity is characteristic of the upper eyelid glands in male Norway rats, while in female black rats, the level of activity is moderate. The activity of ACP in the anal glands shows a moderate level in male Norway rats and an average level in female black rats. ACP in the eccrine glands of the soles of the paws is characterized by a low level of activity in male Norway rats and an average activity in female black rats.
The activity of ALP in the skin glands of the nape has an average level in male Norway rats and a moderate activity in female black rats. ALP activity for the glands of the mouth corners is medium, and for the glands of the upper eyelids — moderate levels in both male Norway rats and female black rats. ALP activity in the anal glands is characterized by a high level in male Norway rats and an average level in female black rats. ALP activity in the eccrine glands of the soles of the paws is high in male Norway rats and moderate in female black rats. The activity of ALP in the sebaceous glands of the soles of the paws has a moderate level in male Norway rats and an average level in female black rats.
ATPase activity does not reach high values in any of the topographical areas of the skin in both male Norway rats and female black rats. ATPase activity in the skin glands of the nape is low in male Norway rats and indistinct in female black rats. The glands of the mouth corners differ in that they show moderate ATPase activity in male Norway rats, while in female black rats, ATPase is not detected; that is, there is no activity. In both species of rats, the upper eyelid glands are characterized by a moderate level of ATPase activity. ATPase activity in the anal glands has an average level in male Norway rats and a moderate level in female black rats. The eccrine glands of the soles of the paws are characterized by an average level of ATPase activity in male Norway rats and low activity in female black rats. The sebaceous glands of the soles of the paws have a low level of ATPase activity in male Norway rats and a moderate level of activity in female black rats.
The activity of peroxidase is the lowest among the other studied enzymes. Thus, in male Norway rats and female black rats, the glands in the nape do not detect peroxidase (there is any activity). Low and indistinct peroxidase activity is shown in the glands of the mouth corners and the eccrine glands of the soles of the paws in male Norway rats and in female black rats, respectively. The glands of the upper eyelids show a low level of peroxidase activity in male Norway rats and no activity in female black rats. The anal glands in male Norway rats are characterized by a lack of peroxidase activity, while in female black rats, this enzyme has indistinct activity. For the sebaceous glands of the soles of the paws of male Norway rats, indistinct peroxidase activity is characteristic, and for female black rats-no activity.
Based on the available digital analogs of the histoenzymatic activity of the skin glands of male Norway rats and female black rats, it is advisable to create two data arrays then find the difference between them. The difference found reflects the interspecific parity (el=0), the dominance of male Norway rats (el > 0), or the dominance of female black rats (el < 0). To do this, the above three outcomes were provided in the "for" loop. The source code has the following sequence of actions:
.. code:: ipython3 import numpy as np
.. code:: ipython3
male_Norway_rats=np.array(
[
[3, 4, 2, 0], [3, 4, 3, 2], [4, 3, 3, 2], [3, 5, 4, 0], [2, 5, 4, 2], [3, 3, 2, 1] ]) male_Norway_rats
.. parsed-literal:: array([[3, 4, 2, 0], [3, 4, 3, 2], [4, 3, 3, 2], [3, 5, 4, 0], [2, 5, 4, 2], [3, 3, 2, 1]])
.. code:: ipython3
female_Black_rats=np.array(
[
[3, 3, 1, 0], [3, 4, 0, 1], [3, 3, 3, 0], [4, 4, 3, 1], [4, 3, 2, 1], [3, 4, 3, 0] ]) female_Black_rats
.. parsed-literal:: array([[3, 3, 1, 0], [3, 4, 0, 1], [3, 3, 3, 0], [4, 4, 3, 1], [4, 3, 2, 1], [3, 4, 3, 0]])
.. code:: ipython3
male_Norway_rats - female_Black_rats
.. parsed-literal:: array([[ 0, 1, 1, 0], [ 0, 0, 3, 1], [ 1, 0, 0, 2],
[-1, 1, 1, -1], [-2, 2, 2, 1], [ 0, -1, -1, 1]])
.. code:: ipython3
for el in (male_Norway_rats - female_Black_rats).flat: if el == 0:
print("Interspecific parity (el=0)") elif el > 0:
print("Dominance of male Norway rats (el > 0)") elif el < 0:
print("Dominance of female Black rats (el < 0)")
.. parsed-literal:: Interspecific parity (el=0)
Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Interspecific parity (el=0)
Interspecific parity (el=0) Interspecific parity (el=0)
Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Interspecific parity (el=0)
Interspecific parity (el=0)
Dominance of male Norway rats (el > 0) Dominance of female Black rats (el < 0) Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Dominance of female Black rats (el < 0) Dominance of female Black rats (el < 0) Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Dominance of male Norway rats (el > 0) Interspecific parity (el=0)
Dominance of female Black rats (el < 0) Dominance of female Black rats (el < 0) Dominance of male Norway rats (el > 0)
The resulting data can be presented in the form of three outcomes with a certain frequency of occurrence. For this purpose, it is advisable to present using a polygon (Figure 5).
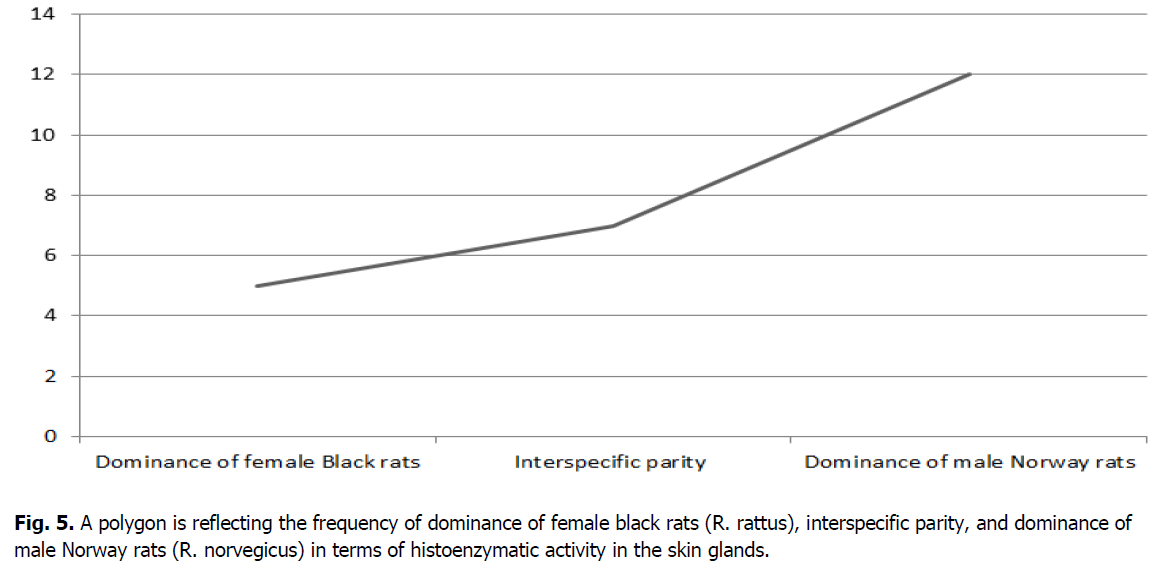
Figure 5: A polygon is reflecting the frequency of dominance of female black rats (R. rattus), interspecific parity, and dominance of male Norway rats (R. norvegicus) in terms of histoenzymatic activity in the skin glands.
Based on the above data, it can be seen that in five cases (20.8%), the histoenzymatic activity of the skin glands prevails in female black rats. In male Norway rats, the dominance of the histochemical parameters of the skin glands amounted to twelve cases (50.0%). The parity ratio indicating the equality of the values of the histoenzymatic activity of the skin glands in male Norway and female black rats amounted to seven cases (29.2%). Thus, the data obtained indicate the prevalence of histoenzymatic activity of the skin glands of male Norway rats over female black rats.
Female Norway rats and male black rats
A comparison of the histoenzymatic activity of the skin glands of female Norway rats and male black rats is shown in Table 3.
| Skin glands | ACP, signs/points |
ALP, signs/points |
ATPase, signs/points |
Peroxidase, signs/points |
||||
|---|---|---|---|---|---|---|---|---|
| Female Norway rats | Female black rats | Female Norway rats | Female black rats | Female Norway rats | Female black rats | Female Norway rats | Female black rats | |
| Nape | +/3 | ++/4 | +/3 | -(+)/1 | +/3 | -(+)/1 | -/0 | -/0 |
| Mouth corners | +/3 | ++/4 | +++/5 | +/3 | ++/4 | -/0 | -/0 | -/0 |
| Upper eyelids | +/3 | ++/4 | +/3 | +/3 | +/3 | +/3 | -/0 | -/0 |
| Anal area | ++/4 | +++/5 | ++/4 | +/3 | ++/4 | ++/4 | -/0 | -/0 |
| Eccrine glands of the soles of the paws | +/3 | -(+)/1 | ++/4 | ++/4 | +/3 | -(+)/1 | +(-)/2 | -/0 |
| Sebaceous glands of the soles of the paws | ++/4 | ++/4 | ++/4 | +/3 | +/3 | +(-)/2 | -/0 | -(+)/1 |
Table 3. Comparative analysis of the histoenzymatic activity of the skin glands of female Norway rats (R. norvegicus) and male black rats (R. rattus)
The activity of ACP in the skin glands of the nape, the mouth corners, and the upper eyelids is moderate in female Norway rats and moderate in male black rats. The activity of ACP in the anal glands shows an average level in female Norway rats and a high level in male black rats. ACP in the eccrine glands of the soles of the paws is characterized by a moderate level of activity in female Norway rats and indistinct activity in male black rats. The average activity of ACP for both species of rats is characteristic of the sebaceous glands of the soles of the paws.
The activity of ALP in the skin glands of the nape has a moderate level in female Norway rats and an indistinct nature in male black rats. ALP activity for the glands of the mouth corners is characterized by a high level for female Norway rats and a moderate level for male black rats. Moderate activity of ALP is typical for both the glands of the upper eyelids and the eccrine glands of the soles of the paws in both species of rats. ALP activity in the anal glands differs, with an average level in female Norway rats and a moderate level in male black rats. The activity of ALP in the sebaceous glands of the soles of the paws has an average level in female Norway rats and a moderate level in male black rats.
ATPase activity does not reach high values in any of the topographical areas of the skin in both female Norway rats and male black rats. ATPase activity in the skin glands of the nape has a moderate level in female Norway rats and an indistinct level in male black rats. The glands of the mouth corners differ in that they show an average activity of ATPase in female Norway rats, while in male black rats, ATPase is not detected; that is, there is no activity. In both species of rats, the upper eyelid glands have a moderate level of ATPase activity, and the anal glands have an average level of activity. ATPase activity in the eccrine glands of the soles of the paws has a moderate level in female Norway rats and an indistinct level in male black rats. The sebaceous glands of the soles of the paws are characterized by a moderate level of ATPase activity in female Norway rats and low activity in male black rats.
The activity of peroxidase is the lowest among the other studied enzymes. Thus, in female Norway rats and male black rats, almost all the glands from the studied topographic areas do not detect peroxidase (lack of activity). Low peroxidase activity occurs only in the eccrine glands of the soles of the paws of female Norway rats, while indistinct activity is characteristic of the sebaceous glands of the soles of the paws of male black rats.
Based on the available digital analogs of the histoenzymatic activity of the skin glands of female Norway rats and male black rats, it is advisable to create two data arrays then find the difference between them. The difference found reflects the interspecific parity (el=0), the dominance of female Norway rats (el > 0), or the dominance of male black rats (el < 0). To do this, the above three outcomes were provided in the "for" loop. The source code has the following sequence of actions:
.. code:: ipython3 import numpy as np
.. code:: ipython3
female_Norway_rats=np.array(
[
[3, 3, 3, 0], [3, 5, 4, 0],
[3, 3, 3, 0], [4, 4, 4, 0], [3, 4, 3, 2], [4, 4, 3, 0] ]) female_Norway_rats
.. parsed-literal:: array([[3, 3, 3, 0], [3, 5, 4, 0], [3, 3, 3, 0], [4, 4, 4, 0], [3, 4, 3, 2], [4, 4, 3, 0]])
.. code:: ipython3
male_Black_rats=np.array(
[
[4, 1, 1, 0], [4, 3, 0, 0], [4, 3, 3, 0], [5, 3, 4, 0], [1, 4, 1, 0], [4, 3, 2, 1] ]) male_Black_rats
.. parsed-literal:: array([[4, 1, 1, 0], [4, 3, 0, 0], [4, 3, 3, 0], [5, 3, 4, 0], [1, 4, 1, 0], [4, 3, 2, 1]])
.. code:: ipython3
female_Norway_rats - male_Black_rats
.. parsed-literal:: array([[-1, 2, 2, 0], [-1, 2, 4, 0], [-1, 0, 0, 0], [-1, 1, 0, 0], [ 2, 0, 2, 2], [ 0, 1, 1, -1]])
.. code:: ipython3
for el in (female_Norway_rats - male_Black_rats).flat: if el == 0:
print("Interspecific parity (el=0)") elif el > 0:
print("Dominance of female Norway rats (el > 0)") elif el < 0:
print("Dominance of male Black rats (el < 0)")
.. parsed-literal::
Dominance of male Black rats (el < 0)
Dominance of female Norway rats (el > 0)
Dominance of female Norway rats (el > 0)
Interspecific parity (el=0)
Dominance of male Black rats (el < 0)
Dominance of female Norway rats (el > 0)
Dominance of female Norway rats (el > 0)
Interspecific parity (el=0)
Dominance of male Black rats (el < 0)
Interspecific parity (el=0)
Interspecific parity (el=0)
Interspecific parity (el=0)
Dominance of male Black rats (el < 0)
Dominance of female Norway rats (el > 0)
Interspecific parity (el=0)
Interspecific parity (el=0)
Dominance of female Norway rats (el > 0)
Interspecific parity (el=0)
Dominance of female Norway rats (el > 0)
Dominance of female Norway rats (el > 0)
Interspecific parity (el=0)
Dominance of female Norway rats (el > 0)
Dominance of female Norway rats (el > 0)
Dominance of male Black rats (el < 0)
The resulting data can be presented in the form of three outcomes with a certain frequency of occurrence. For this purpose, it is advisable to present using a polygon (Figure 6).
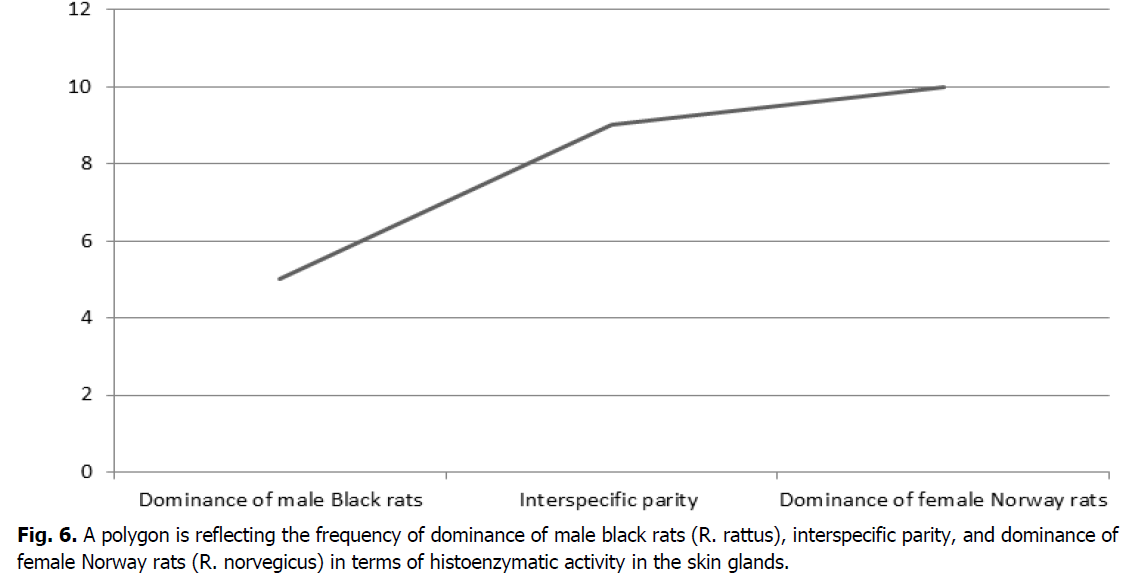
Figure 6: A polygon is reflecting the frequency of dominance of male black rats (R. rattus), interspecific parity, and dominance of female Norway rats (R. norvegicus) in terms of histoenzymatic activity in the skin glands.
Female Norway rats and female black rats
A comparison of the histoenzymatic activity of the skin glands of female Norway rats and female black rats is shown in Table 4.
| Skin glands | ACP, signs/points |
ALP, signs/points |
ATPase, signs/points |
Peroxidase, signs/points |
||||
|---|---|---|---|---|---|---|---|---|
| Female Norway rats | Female black rats | Female Norway rats | Female black rats | Female Norway rats | Female black rats | Female Norway rats | Female black rats | |
| Nape | +/3 | +/3 | +/3 | +/3 | +/3 | -(+)/1 | -/0 | -/0 |
| Mouth corners | +/3 | +/3 | +++/5 | ++/4 | ++/4 | -/0 | -/0 | -(+)/1 |
| Upper eyelids | +/3 | +/3 | +/3 | +/3 | +/3 | +/3 | -/0 | -/0 |
| Anal area | ++/4 | ++/4 | ++/4 | ++/4 | ++/4 | +/3 | -/0 | -(+)/1 |
| Eccrine glands of the soles of the paws | +/3 | ++/4 | ++/4 | +/3 | +/3 | +(-)/2 | +(-)/2 | -(+)/1 |
| Sebaceous glands of the soles of the paws | ++/4 | +/3 | ++/4 | ++/4 | +/3 | +/3 | -/0 | -/0 |
Table 4. Comparative analysis of the histoenzymatic activity of the skin glands of female Norway rats (R. norvegicus) and female black rats (R. rattus).
Based on the above data, it can be seen that in five cases (20.8%), the histoenzymatic activity of the skin glands prevails in male black rats. In female Norway rats, the dominance of the histochemical parameters of the skin glands amounted to ten cases (41.7%). The parity ratio indicating the equality of the values of the histoenzymatic activity of the skin glands in female Norway and male black rats amounted to nine cases (37.5%). Thus, the data obtained indicate the prevalence of histoenzymatic activity of the skin glands of female Norway rats over male black rats.
The activity of ACP does not reach high values in any of the topographical areas of the skin in both female Norway rats and female black rats. The activity of ACP in the skin glands of the nape, the mouth corners, and upper eyelids is moderate, and in the anal glands — an average level in both species of rats. ACP in the eccrine glands of the soles of the paws is characterized by a moderate level of activity in female Norway rats and an average level of activity in female black rats. ACP in the sebaceous glands of the soles of the paws is characterized by an average level of activity in female Norway rats and a moderate level of activity in female black rats.
ALP activity in the skin glands of the nape and upper eyelids has a moderate level, and in the anal glands and in the sebaceous glands of the soles of the paws — an average level in both species of rats. ALP activity in the glands of the mouth corners is characterized by a high level in female Norway rats and an average level in female black rats. ALP activity in the eccrine glands of the soles of the paws has an average level in female Norway rats and a moderate level in female black rats.
ATPase activity does not reach high values in any of the topographical areas of the skin in both female Norway rats and female black rats. ATPase activity in the skin glands of the nape has a moderate level in female Norway rats and an indistinct level in female black rats. The glands of the mouth corners of the differing show an average activity of ATPase in female Norway rats, while in female black rats, ATPase is not detected; that is, there is no activity. In both species of rats, the glands of the upper eyelids and the sebaceous glands of the soles of the paws are characterized by a moderate level of ATPase activity. ATPase activity in the anal glands has an average level in female Norway rats and a moderate level in female black rats. The eccrine glands of the soles of the paws are characterized by a moderate level of ATPase activity in female Norway rats and low activity in female black rats.
The activity of peroxidase is the lowest among the other studied enzymes. Thus, in female Norway rats and female black rats, the glands in the nape, upper eyelids, and the sebaceous glands of the soles of the paws do not detect peroxidase (no activity). The mouth corners and anal glands' glands show no peroxidase activity in female Norway rats and indistinct activity in female black rats. The eccrine glands of the soles of the paws of female Norway rats are characterized by low peroxidase activity and for female black rats-indistinct activity.
Based on the available digital analogs of the histoenzymatic activity of the skin glands of female Norway rats and female black rats, it is advisable to create two data arrays then find the difference between them. The difference found reflects the interspecific parity (el=0), the dominance of female Norway rats (el > 0), or the dominance of female black rats (el < 0). To do this, the above three outcomes were provided in the "for" loop. The source code has the following sequence of actions:
.. code:: ipython3 import numpy as np
.. code:: ipython3
female_Norway_rats=np.array(
[
[3, 3, 3, 0], [3, 5, 4, 0], [3, 3, 3, 0], [4, 4, 4, 0], [3, 4, 3, 2], [4, 4, 3, 0] ]) female_Norway_rats
.. parsed-literal:: array([[3, 3, 3, 0], [3, 5, 4, 0], [3, 3, 3, 0], [4, 4, 4, 0], [3, 4, 3, 2], [4, 4, 3, 0]])
.. code:: ipython3
female_Black_rats=np.array(
[
[3, 3, 1, 0], [3, 4, 0, 1], [3, 3, 3, 0], [4, 4, 3, 1], [4, 3, 2, 1],
[3, 4, 3, 0] ]) female_Black_rats
.. parsed-literal:: array([[3, 3, 1, 0], [3, 4, 0, 1], [3, 3, 3, 0], [4, 4, 3, 1], [4, 3, 2, 1], [3, 4, 3, 0]])
.. code:: ipython3
female_Norway_rats - female_Black_rats
.. parsed-literal:: array([[ 0, 0, 2, 0], [ 0, 1, 4, -1], [ 0, 0, 0, 0], [ 0, 0, 1, -1], [-1, 1, 1, 1], [ 1, 0, 0, 0]])
.. code:: ipython3
for el in (female_Norway_rats - female_Black_rats).flat: if el == 0:
print("Interspecific parity (el=0)") elif el > 0:
print("Dominance of female Norway rats (el > 0)") elif el < 0:
print("Dominance of female Black rats (el < 0)")
.. parsed-literal::
Interspecific parity (el=0) Interspecific parity (el=0)
Dominance of female Norway rats (el > 0) Interspecific parity (el=0)
Interspecific parity (el=0)
Dominance of female Norway rats (el > 0) Dominance of female Norway rats (el > 0) Dominance of female Black rats (el < 0)
Interspecific parity (el=0) Interspecific parity (el=0) Interspecific parity (el=0) Interspecific parity (el=0) Interspecific parity (el=0) Interspecific parity (el=0)
Dominance of female Norway rats (el > 0) Dominance of female Black rats (el < 0) Dominance of female Black rats (el < 0) Dominance of female Norway rats (el > 0) Dominance of female Norway rats (el > 0) Dominance of female Norway rats (el > 0) Dominance of female Norway rats (el > 0) Interspecific parity (el=0)
Interspecific parity (el=0) Interspecific parity (el=0)
The resulting data can be presented in the form of three outcomes with a certain frequency of occurrence. For this purpose, it is advisable to present using a polygon (Figure 7).
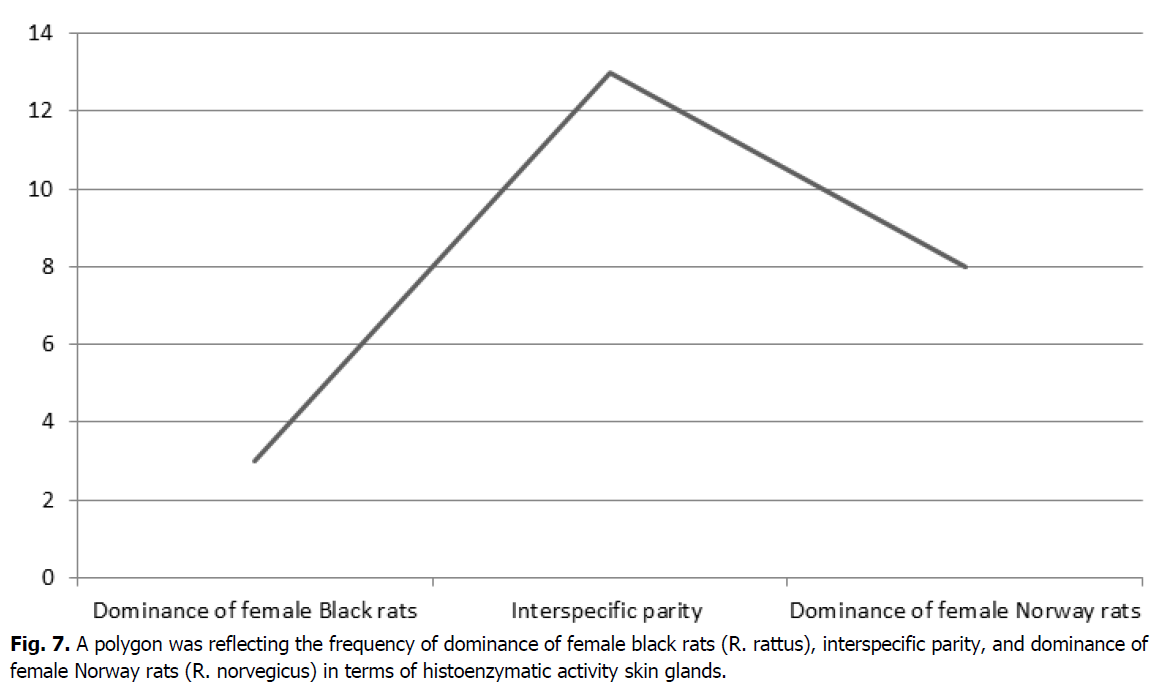
Figure 7: A polygon was reflecting the frequency of dominance of female black rats (R. rattus), interspecific parity, and dominance of female Norway rats (R. norvegicus) in terms of histoenzymatic activity skin glands.
Based on the above data, it can be seen that in three cases (12.5%), the histoenzymatic activity of the skin glands prevails in female black rats. In female Norway rats, the dominance of the histochemical parameters of the skin glands amounted to eight cases (33.3%). The parity ratio indicating the equality of the values of the histoenzymatic activity of the skin glands in female Norway and female black rats amounted to thirteen cases (54.2%). Thus, the data obtained indicate the prevalence of a parity outcome and indicates a certain superiority of the histoenzymatic activity of the skin glands of female Norway rats over female black rats.
Discussion
It is known that enzymes, which are biological catalysts, are responsible for the acceleration of biochemical reactions (Berg et al., 2002) occurring at the cellular level, which is necessary for the active maintenance of intracellular metabolism (Wilson, 1973; Lyon et al., 1991), which, from the point of view of the physiology of the skin glands, is mainly expressed in the activation of secretory formation. This is because the studied enzymes ensure the production and transport of the necessary amount of secretion (Hunter, 1995; Berg et al., 2001), while the "quality" of the secretion can correlate with the current parameters of the individual, which is an example of physiological adaptation (Dzhemukhadze, 2007). The considered variants of interspecific relationships between males and females of Norway and black rats are revealed precisely at the level of marking and social behavior (Barnett, 2017), where the main sources of chemical signals are both sebaceous and eccrine glands (Sokolov & Chernova, 2001), while enzyme systems are among the key factors that provide intracellular activation of biochemical processes (Lyon et al., 1991; Qin et al., 2013).
Discussing the methods used, it is advisable to emphasize the need for a broader application of bioinformatics approaches (Stevens &Boucher, 2015) that allow formalizing and automating the assessment of interspecific interaction between Norway and black rats at the level of identifying the algebraic difference between the elements of arrays of digital analogs of the histoenzymatic activity of the skin glands. For this purpose, we used the Python program (Ekmekci et al., 2016).
Further presentation of the data in the form of constructed polygons reflecting the frequency of occurrence of the three outcomes and finding their percentage ratio allows us to rank the available options, and the data obtained can be presented in the form of a matrix (Table 5).
| Interspecific interactions | Male black rats | Female black rats |
|---|---|---|
| Male Norway rats | Male Norway rats > Interspecific parity | Male Norway rats > Interspecific parity > |
| > Male black rats | Female black rats | |
| Female Norway rats | Female Norway rats > Interspecific | Interspecific parity > Female Norway rats |
| parity > Male black rats | > Female black rats |
Table 5. Ranking of interactions between Norway (R. norvegicus) and black (R. rattus) rats with an indication of interspecific dominance or parity.
The obtained generalized results indicate a general trend in all four variants of interaction: the histoenzymatic activity of the skin glands of Norway rats prevails over the histoenzymatic activity of black rats. In addition, a significant level of interspecific parity is noteworthy, which indicates identical values of the histochemical activity of the skin glands in both Norway and black rats. In contrast, the frequency of occurrence of this outcome in interspecific interaction between females of both species has the maximum value. The results are quite expected, given the taxonomic proximity of Norway and black rats (Milyutin, 1990).
Formally, according to the data obtained, it is possible to speak about the dominance of one rat species over another from the point of view of the physiological basis of marking behavior, since histochemical activity forms the natural conditions for activating intracellular metabolism (Lyon et al., 1991), and, consequently, affects the process of secreting skin glands. However, the results obtained must be treated with a particular assumption because, first, we have built a model of interaction, and secondly, enzymes have an indirect effect on the level of marking animals with skin gland secretions. That is why the higher the frequency of occurrence related to a particular species of rat, the greater the level of interspecific influence they have.
Conclusion
The study performed at the intersection of zoology, histochemistry, and bioinformatics, revealed the level of variability in the histoenzymatic activity of the glands of the skin of Norway and black rats. At the same time, pairwise comparison of the values of histochemical activity, along with the bioinformatic approach used, made it possible to determine the frequency of occurrence of the dominance of one species over another and identify interspecific parity. This approach allows us to understand the significance of histoenzymatic activity, which correlates with both physiological and biochemical parameters of the skin glands of Norway and black rats, and the results obtained expand knowledge about the role of histochemistry of enzymes in various biological processes.
References
Azgaldov, G.G., Kostin, A.V. (2011). Applied qualimetry: its origins, errors and misconceptions. Benchmarking: An International Journal, 18:428-444.
Barnett, S.A. (2017). The Rat: A Study in Behavior. Routledge, New York.
Bell, M. (1986). Sebaceous glands. In Biology of the Integument. Springer, Berlin, Heidelberg, pp. 318-338. Berg, J.M., Tymoczko, J.L., Stryer, L. (2002). Biochemistry (5th ed.). San Francisco:W.H. Freeman and Company. Berg, J.S. (2001). Powell BC, and Cheney RE. A millennial myosin census. Molecular Biology of the Cell, 12:780-794. Burstone, M.S. (1962). Enzyme histo-chemistry and its application in the study of neoplasms. Enzyme histo-chemistry and its application in the study of neoplasms.
Cui, C.Y., Schlessinger, D. (2015). Eccrine sweat gland development and sweat secretion. Experimental dermatology, 24:644-650. Dzhemukhadze, N.K. (2007). The dependence of interspecific differences in the histoenzymatic parameters of skin glands between Norway (Rattus norvegicus) and black (Rattus rattus) rats on their social behavior. In Doklady Biological Sciences, 416:368.
Dzhemukhadze, N.K., Kiladze, A.B. (2008, December). Comparison of the activity of some phosphatases in the midventral gland and nonspecific sebaceous glands of the neck in the Campbell hamster (Phodopus campbelli). In Doklady Biological Sciences, 423:447. Ekmekci, B., McAnany, C. E., Mura, C. (2016). An introduction to programming for bioscientists: a Python-based primer. PLoS Computational Biology, 12:e1004867.
Feng, A.Y., Himsworth, C.G. (2014). The secret life of the city rat: a review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosystems, 17:149-162.
Foster, S.P. (2010). Interspecific competitive interactions between Rattus norvegicus and R. rattus (Doctoral dissertation, The University of Waikato).
Hashimoto, K., Hori, K., Aso, M. (1986). Sweat glands. In Biology of the Integument. Springer, Berlin, Heidelberg, pp:339-356. Hunter, T. (1995). Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell, 80:225-236. Kalinin, A.A. (1995). Mezhvidovye otnosheniya seryh i chernyh krys [Interspecific relationships between Norway and black rats]. RAN, Moscow (in Russian).
Kiladze, A.B., Dzhemukhadze, N.K. (2019). Evaluation of sexual dimorphism of histochemical activity of phosphatases of the plantar glands of Norway rats (Rattus norvegicus). Biosystems Diversity, 27:39-42.
Kiladze, A.B. Dzhemukhadze, N.K. (2020). Biokvalimetricheskij analiz aktivnosti fosfataz zhelez kozhnogo pokrova seryh i chernyh krys [Bioqualimetric study of the phosphatases activity of skin glands of Norway rats and black rats]. Institute of Computer Science, Moscow, Izhevsk. (In Russian).
King, C.M., Foster, S., Miller, S. (2011). Invasive European rats in Britain and New Zealand: same species, different outcomes. Journal of Zoology, 285:172-179.
Lojda, Z., Gossrau, R., Schiebler, T. (1979). Enzyme Histochemistry. A Labo-ratory Manual.
Lyon, H., Barer, M.R. (1991). The Scope of Histochemistry. In Theory and Strategy in Histochemistry, pp:3-6.
Lyon, H., van Deurs, B., Prentø, P., Hasselager, E., Schulte, E. (1991). The Structural and Chemical Basis for Histochemistry. In Theory and Strategy in Histochemistry, pp:7-31.
Milyutin, A.I. (1990). Sistematika [Systematics]. In: Sokolov, V. E. and Karasjova, E.V. (eds). Seraya krysa: Sistematika, ekologiya,
reguliatsiya chislennosti [Norway rat: Systematics, Ecology, Population Control]. Nauka, Moscow, pp:7-33.
Moseley, H.N.B. (2020). Statistical Methods for Biologists. In: Baxevanis, A.D., Bader, G.D., Wishart, D.S. (eds). Bioinformatics. Fourth Edition. John Wiley & Sons, Inc., Chennai, pp:555-582.
Pears, A.G.E. (1960). Histochemistry:Theoretical and Applied. 2nd ed. J. and A. Churchill Ltd., London.
Pereira, V., Cruz, F., Rocha, M. (2021). MEWpy: a computational strain optimization workbench in Python. Bioinformatics.
Qin, S., Hu, C., Oenema, O. (2013). Differentiating intracellular from extracellular alkaline phosphatase activity in soil by sonication. PloS one, 8:e58691.
Quay, W.B. (1986). Scent glands. In Biology of the Integument, pp:357-373.
Saga, K. (2001). Histochemical and immunohistochemical markers for human eccrine and apocrine sweat glands: an aid for histopathologic differentiation of sweat gland tumors. In Journal of Investigative Dermatology Symposium Proceedings, 6:49-53. Saga, K. (2002). Structure and function of human sweat glands studied with histochemistry and cytochemistry. Progress in Histochemistry and Cytochemistry, 37:323-386.
Saga, K., Morimoto, Y. (1995). Ultrastructural localization of alkaline phosphatase activity in human eccrine and apocrine sweat glands. Journal of Histochemistry & Cytochemistry, 43:927-932.
Schweinfurth, M.K. (2020). The social life of Norway rats (Rattus norvegicus). Elife, 9:e54020.
Sokolov, V.E., Chernova, O.F. (2001). Kozhnye zhelezy mlekopitayushchih [Mammalian skin glands]. Geos, Moscow (in Russian). Stevens, T.J., Boucher, W. (2015). Python Programming for Biology. Cambridge University Press.
Van Noorden, C.J., Frederiks, W.M. (1992). Enzyme histochemistry: a laboratory manual of current methods. Oxford University Press, USA.
Wilson, P.D. (1973). Enzyme changes in ageing mammals. Gerontology, 19:79-125.
Author Info
A.B. Kiladze* and N.K. DzhemukhadzeCitation: Kiladze, A.B., Dzhemukhadze, N.K.. (2021). Bioinformatics model of histoenzymatic parameters of the skin glands of Norway (Rattus norvegicus) and black (Rattus rattus) rats. Ukrainian Journal of Ecology, 11 (5), 51-65.
Received: 22-Jun-2021 Accepted: 02-Jul-2021 Published: 30-Jul-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
